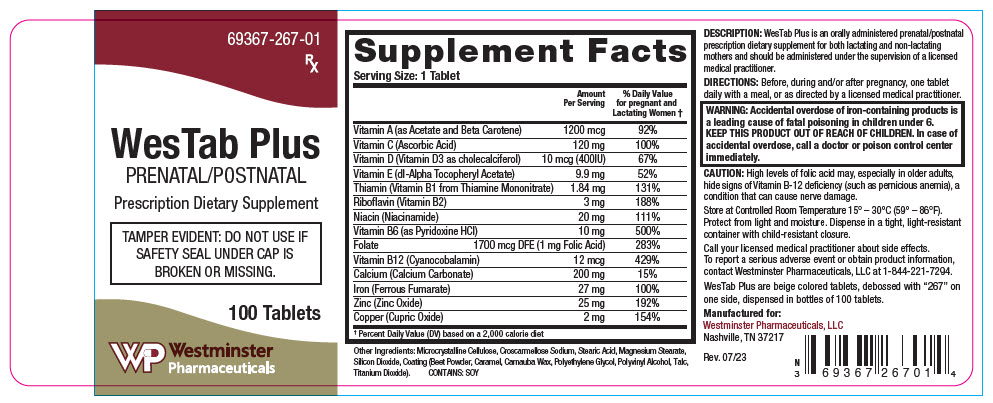
| Supplement Facts | ||
|---|---|---|
| Serving Size: 1 Tablet | ||
| Amount Per Serving | % Daily Value for pregnant and Lactating Women * | |
|
||
| Vitamin A (as Acetate and Beta Carotene) | 1200 mcg | 92% |
| Vitamin C (Ascorbic Acid) | 120 mg | 100% |
| Vitamin D (Vitamin D3 as cholecalciferol) | 10 mcg (400IU) | 67% |
| Vitamin E (dl-Alpha Tocopheryl Acetate) | 9.9 mg | 52% |
| Thiamin (Vitamin B1 from Thiamine Mononitrate) | 1.84 mg | 131% |
| Riboflavin (Vitamin B2) | 3 mg | 188% |
| Niacin (Niacinamide) | 20 mg | 111% |
| Vitamin B6 (as Pyridoxine HCl) | 10 mg | 500% |
| Folate | 1700 mcg DFE (1 mg Folic Acid) | 283% |
| Vitamin B12 (Cyanocobalamin) | 12 mcg | 429% |
| Calcium (Calcium Carbonate) | 200 mg | 15% |
| Iron (Ferrous Fumarate) | 27 mg | 100% |
| Zinc (Zinc Oxide) | 25 mg | 192% |
| Copper (Cupric Oxide) | 2 mg | 154% |
Other Ingredients: Microcrystalline Cellulose, Croscarmellose Sodium, Stearic Acid, Magnesium Stearate, Silicon Dioxide, Coating (Beet Powder, Caramel, Carnauba Wax, Polyethylene Glycol, Polyvinyl Alcohol, Talc, Titanium Dioxide).
CONTAINS: SOY
DESCRIPTION
WesTab Plus is an orally administered prenatal/postnatal prescription dietary supplement for both lactating and non-lactating mothers and should be administered under the supervision of a licensed medical practitioner.
DIRECTIONS
Before, during and/or after pregnancy, one tablet daily with a meal, or as directed by a licensed medical practitioner.
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
CAUTION
High levels of folic acid may, especially in older adults, hide signs of Vitamin B-12 deficiency (such as pernicious anemia), a condition that can cause nerve damage.
Store at Controlled Room Temperature 15° – 30°C (59° – 86°F). Protect from light and moisture. Dispense in a tight, light-resistant container with child-resistant closure.
Call your licensed medical practitioner about side effects. To report a serious adverse event or obtain product information, contact Westminster Pharmaceuticals, LLC at 1-844-221-7294.
WesTab Plus are beige colored tablets, debossed with "267" on one side, dispensed in bottles of 100 tablets.