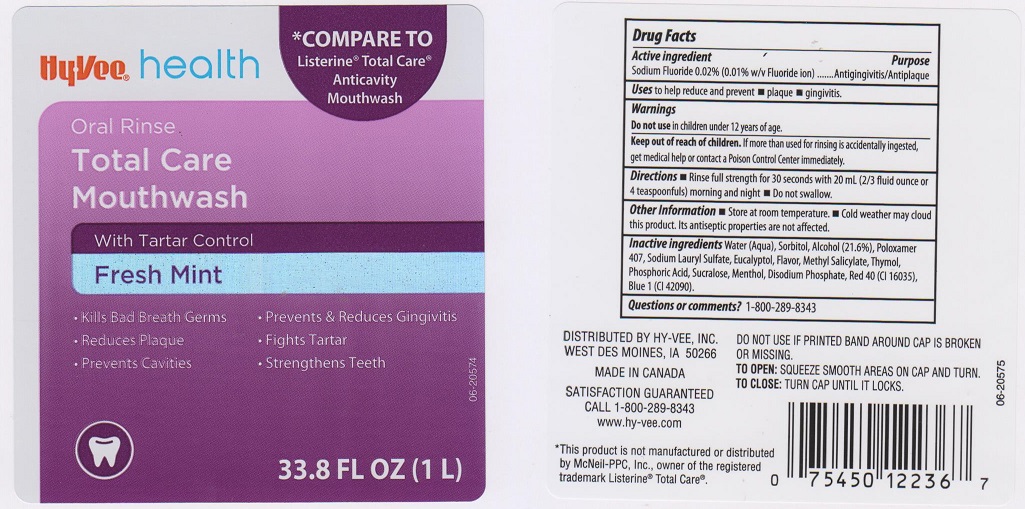
HYVEE COMPLETE CARE FRESH MINT- sodium fluoride liquid
HYVEE INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Sodium Fluoride 0.02% (0.01% w/v Fluoride ion)
Purpose
Antigingivitis/Antiplaque
Uses
to help reduce and prevent
Warnings
Do not use in children under 12 years of age
Keep out of reach of children
If more than used for rinsing is accidentally ingested, get medical help or contact a Poison Control Center immediately
Directions
- Rinse full strength for 30 seconds with 20 mL (2/3 fluid ounce or 4 teaspoonfuls) morning and night
- Do not swallow
Other information
- Store at room temperature
- Cold weather may cloud this product. Its antiseptic properties are not affected.
Inactive ingredients
Water (Aqua), Sorbitol, Alcohol (21.6%), Poloxamer 407, Sodium Lauryl Sulfate, Eucalyptol, Flavor, Methyl Salicylate, Thymol, Phosphoric Acid, Sucralose, Menthol, Disodium Phosphate, Red 40 (CI 16035), Blue 1 (CI 42090).
Questions or comments?
1-800-289-8343
Label Copy
HYVEE INC.
