FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
ICG for Injection Set, a tricarbocyanine dye, is indicated for use with the KARL STORZ ICG Imaging System to provide real-time endoscopic visible and near-infrared fluorescence imaging. Indocyanine Green for Injection, USP used in conjunction with the KARL STORZ ICG Imaging System enables surgeons to perform minimally invasive surgery using standard endoscopic visible light as well as:
1.2 Visual assessment of at least one of the major extra-hepatic bile ducts (cystic duct, common bile duct and common hepatic duct), using near-infrared imaging.
Visual assessment of at least one of the major extra-hepatic bile ducts (cystic duct, common bile duct and common hepatic duct), using near-infrared imaging. Fluorescence imaging of biliary ducts with the KARL STORZ ICG Imaging System is intended for use with standard of care white light and, when indicated, intraoperative cholangiography. The device is not intended for standalone use for biliary duct visualization.
2 DOSAGE AND ADMINISTRATION
2.1 Perfusion Assessment
Preparation of ICG for Administration
Under sterile conditions, reconstitute one (1) 25 mg vial of Indocyanine Green for Injection, USP using one (1) 10 mL Sterile Water for Injection, USP vial located in the ICG for Injection Set. Shake the ICG vial gently to dissolve. After reconstitution, a 25 mg vial of ICG contains 2.5 mg of dye per mL of solution, so a 1.0 mL injection contains a 2.5 mg dose of ICG.
Indocyanine Green for Injection, USP must be used within 6 hours after reconstitution. If a precipitate is present, discard the solution.
Dosage
A 3 mL (7.5 mg) dose followed by a 10 mL bolus of saline is recommended. Multiple doses can be administered as required, up to the maximum recommended dose.
Maximum recommended dose
The total dose of dye injected should be kept below 2 mg/kg.
Timing of ICG Administration
ICG fluorescence is quickly visible within blood vessels, tissue and organs, and it does not last very long (refer to Table 1 below).
|
Blood Vessels |
Organs (Kidney, Liver, Adrenal Gland, Small Bowel) |
|
|
See within: |
5-30 seconds |
1-2 minutes |
|
Visibility lasts: |
20-30 seconds |
20-120 minutes |
For fluorescence imaging of PERFUSION in blood vessels, administration of the ICG should be performed at the time fluorescence imaging is requested by the physician. Multiple imaging sequences may be performed as necessary [up to the maximum dose (2 mg/kg of patient body weight)], so it is recommended to withdraw the desired dosage of ICG solution for each planned imaging sequence into separate syringes ahead of time.
Method of Administration
ICG administration is to be performed via a central or peripheral venous line. Inject the prepared dose of ICG solution into the central or peripheral line as a tight bolus and immediately followed by a bolus of 10-12 mL of normal saline for injection.
2.2 Extra-Hepatic Biliary Anatomy
Preparation of ICG for Administration
Under sterile conditions, reconstitute one (1) 25 mg vial of Indocyanine Green for Injection, USP using one (1) 10 mL Sterile Water for Injection, USP vial located in the ICG for Injection Set. Shake the ICG vial gently to dissolve. After reconstitution, a 25 mg vial of ICG contains 2.5 mg of dye per mL of solution, so a 1.0 mL injection contains a 2.5 mg dose of ICG.
Indocyanine Green for Injection, USP must be used within 6 hours after reconstitution. If a precipitate is present, discard the solution.
Dosage
A 0.02 mL/kg dose that is scaled to the patient’s weight is recommended. This provides 0.05 mg/kg of ICG.
Maximum recommended dose
The total dose of dye injected should be kept below 2 mg/kg.
Timing of ICG Administration
Following intravenous injection, ICG is rapidly bound to plasma protein, of which albumin is the principle carrier (95%). ICG is taken up from the plasma almost exclusively by the hepatic parenchymal cells and is secreted entirely into the bile. For optimal fluorescence imaging of Extra-Hepatic Biliary Anatomy, ICG should be administered at least 45 minutes prior to the time fluorescence imaging is desired by the physician. If this preoperative administration is not performed, however, ICG can be administered once the patient is in the OR, as adequate fluorescence imaging is possible in as little as 15 minutes after IV injection.
Method of Administration
ICG administration is to be performed via a central or peripheral venous line. Inject the prepared weight-scaled dose of ICG solution into the central or peripheral line as a tight bolus and immediately followed by a bolus of 10-12 mL of normal saline for injection.
3 DOSAGE FORMS AND STRENGTHS
Indocyanine Green for Injection, USP is a sterile, lyophilized green powder containing 25 mg of indocyanine green with no more than 5% sodium iodide.
4 CONTRAINDICATIONS
Indocyanine Green for Injection, USP contains sodium iodide and should be used with caution in patients who have a history of allergy to iodides because of the risk of anaphylaxis.
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis
Deaths from anaphylaxis have been reported following Indocyanine Green for Injection, USP administration during cardiac catheterization.
5.2 Drug Instability
Indocyanine Green for Injection, USP is unstable in aqueous solution and must be used within 6 hours. However, the dye is stable in plasma and whole blood so that samples obtained in discontinuous sampling techniques may be read hours later. Sterile techniques should be used in handling the dye solution as well as in the performance of the procedures. If a precipitate is present, discard the solution.
6 ADVERSE REACTIONS
Anaphylactic or urticarial reactions have been reported in patients with or without history of allergy to iodides. If such reactions occur, treat with the appropriate agents, e.g., epinephrine, antihistamines, and corticosteroids.
7 DRUG INTERACTIONS
Preparations containing sodium bisulfite, including some heparin products reduce the absorption peak of Indocyanine Green for Injection, USP in blood and, therefore, should not be used as an anticoagulant for the collection of samples for analysis.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Animal reproduction studies have not been conducted with Indocyanine Green for Injection, USP. It is also not known whether Indocyanine Green for Injection, USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Indocyanine Green for Injection, USP should be given to a pregnant woman only if clearly indicated.
10 OVERDOSAGE
There are no data available describing the signs, symptoms, or laboratory findings accompanying overdosage. The LD50 after intravenous administration ranges between 60 and 80 mg/kg in mice, 50 and 70 mg/kg in rats and 50 and 80 mg/kg in rabbits.
11 DESCRIPTION
Indocyanine Green for Injection, USP is a sterile, lyophilized green powder containing 25 mg of indocyanine green with no more than 5% sodium iodide. It is packaged with Sterile Water for Injection, USP used to dissolve the indocyanine green. Indocyanine Green for Injection, USP is to be administered intravenously.
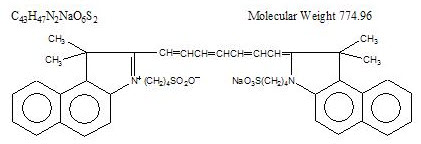
Indocyanine green is a water soluble, tricarbocyanine dye with a peak spectral absorption at 800 nm. The chemical name for Indocyanine Green is 1 H-Benz[e]indolium, 2-[7-[1,3-dihydro-1,1-dimethyl-3-(4-sulfobutyl)-2H-benz[e] indol-2-ylidene]-1,3,5-heptatrienyl]-1,1-dimethyl-3-(4-sulfobutyl)-,hydroxide, inner salt, sodium salt. Indocyanine Green for Injection, USP has a pH of approximately 6.5 when reconstituted. Each vial of Indocyanine Green for Injection, USP contains 25 mg of indocyanine green as a sterile lyophilized powder.
12 CLINICAL PHARMACOLOGY
Following intravenous injection, Indocyanine Green for Injection, USP is rapidly bound to plasma protein, of which albumin is the principle carrier (95%). Indocyanine Green for Injection, USP undergoes no significant extrahepatic or enterohepatic circulation; simultaneous arterial and venous blood estimations have shown negligible renal, peripheral, lung or cerebro-spinal uptake of the dye. Indocyanine Green for Injection, USP is taken up from the plasma almost exclusively by the hepatic parenchymal cells and is secreted entirely into the bile.
The peak absorption and emission of Indocyanine Green for Injection, USP lie in a region (800 to 850 nm) where transmission of energy by the pigment epithelium is more efficient than in the region of visible light energy. Indocyanine Green for Injection, USP also has the property of being nearly 98% bound to blood protein.
16 HOW SUPPLIED/STORAGE AND HANDLING
ICG for Injection Set is a kit (NDC 70599-424-02) containing one Indocyanine Green for Injection, USP kit (NDC 70100-424-02) and these Instructions For Use with the KARL STORZ ICG Imaging System.
The Indocyanine Green for Injection, USP kit (NDC 70100-424-02) contains six 25 mg Indocyanine Green for Injection, USP vials and six 10 mL Sterile Water for Injection, USP plastic vials:
NDC 70100-424-01 Indocyanine Green for Injection, USP vial. 25 mg fill in 25 mL vial.
NDC 63323-185-10 (or NDC 0409-4887-17) Sterile Water for Injection, USP, 10 mL fill in 10 mL plastic vials.
ICG for Injection Set
Distributed by:
KARL STORZ Endoscopy-America, Inc.
El Segundo, CA 90245 USA
50441
PRINCIPAL DISPLAY PANEL - KIT CARTON
Front Panel
NDC 70599-424-02 PN: VTG0001
ICG for Injection Set
For Intravenous Administration 25 mg/Vial Kit Rx Only - Sterile
STORZ
KARL STORZ-ENDOSKOPE
Back Panel
NDC 70599-424-02 PN: VTG0001
ICG for Injection Set
Contents:
Six Indocyanine Green for Injection, USP vials (25 mg each)
Six Sterile Water for Injection, USP Vials (10 mL each)
Lot Code Area
No Print / No Coating
STORZ
KARL STORZ-ENDOSKOPE
For Intravenous Administration 25 mg/Vial Kit Rx Only - Sterile 06/2016
Left Panel
ICG for Injection Set
DIRECTIONS FOR USE ENCLOSED
CAUTION: To ensure accurate readings,
Indocyanine Green, USP dissolved in Sterile Water
for Injection, USP must be used within 6 hours.
STORAGE: Store at 20° to 25°C (68° to 77°F)
[See USP Controlled Room Temperature].
USAGE: See package insert for dosage information.
25 mg/Vial Kit
Right Panel
ICG for Injection Set
Distributed by:
KARL STORZ
Endoscopy-America, Inc.
2151 E. Grand Ave.
El Segundo, CA 90245
Phone: (800) 421-0837
Fax: (800) 321-1304
E-Mail: communications@karlstorz.com
Web: www.karlstorz.com
Rx Only
25 mg/Vial Kit
PRINCIPAL DISPLAY PANEL - VIAL
NDC 70100-424-01
Indocyanine Green
for Injection, USP
25 mg/Vial
For Intravenous Administration
After reconstitution, use within 6 hours.
Rx only Sterile
Distributed by Diagnostic Green LLC
50428
Lot. No.
Exp.
PRINCIPAL DISPLAY PANEL - STERILE WATER VIAL
10 mL Single-dose
Sterile Water
for Injection, USP
FOR DRUG DILUENT USE
Rx only NDC 0409-4887-17
Contains no antimicrobial or other added
substance. Sterile, nonpyrogenic. Do not give
intravenously unless rendered nearly isotonic.
Hospira, Inc. RL-4428
Lake Forest, IL 60045 USA