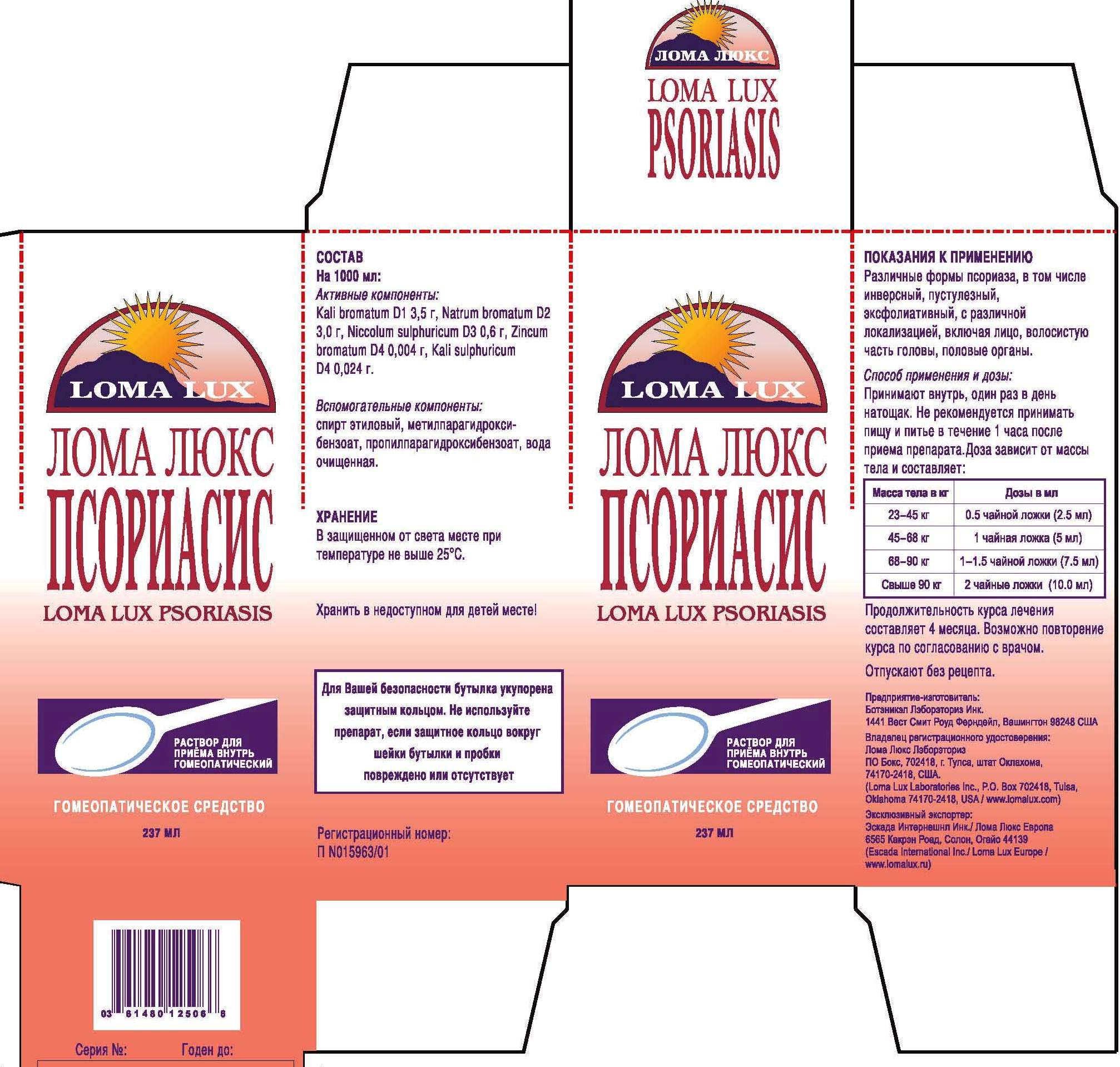
ACTIVE INGREDIENTS: HPUS Homeopathic Liquid - Kali bromatum 1X, Natrum bromatum 2X, Niccolum sulphuricum 3X, Zincum bromatum 4X, Kali sulphuricum 4X.
WARNINGS: Do not use if pregnant or nursing.
If allergic to nickel or costume jewelry, use only under the advice and supervision of a physician.
If symptoms worsen, contact a physician.
Keep out of reach of children.
CAUTION: Use only as directed.
Do not give to children under ten years old or use in the presence of kidney disease.
If skin rash appears or if nervous symptoms persist, recur frequently or are unusual, discontinue use and consult a physician.
DIRECTIONS: Take medication orally at the beginning of the day before eating or drinking anything other than water. Take nothing but water for one hour after taking medication in order to improve absorption. Continue Loma Lux Psoriasis as long as it is beneficial to your overall condition. Recommended minimum duration for treatment is 4-5 months.
Weight Daily Dosage Bottle Lasts
50-100 lbs 1/2 tsp 96 days
100-150 lbs 1 tsp 48 days
150-200 lbs 1 1/2 tsp 36 days
Over 200 lbs 2 tsp 24 days