Primary Package Legal Copy and Artwork including active ingredients, inactive ingredients, instructions for use

LIDOCAINE 4% PATCH
4% LIDOCAINE PAIN RELIEVING PATCH
4% LIDOCANIA PARCHE PARA ALIVIAR EL DOLOR
4% LIDOCAÏNE TIMBRE DE SOULAGEMENT DE LA DOULEUR
Drug Facts
Active ingredient (in each patch) Purpose
Lidocaine 4%...........................................................Topical anesthetic/analgesic/antipruritic
Inactive ingredients
Crospovidone, menthoxypropanediol, non-woven backing fabric, polyester film, non-latex rubber based adhesive*, vanillyl butyl ether
*adhesive not made with natural rubber latex.
Warnings
Do not apply over damaged, raw or blistered skin.
Do not apply excessive quantity of patches within 24 hour period.
Apply locally as needed; do not apply over large surface area of the body.
Avoid contact with the eyes. If eye contact occurs, rinse thoroughly with water. If condition worsens, or if symptoms persist for more than 7 days
or clear up and occur again within a few days, discontinue use of this product and consult a doctor.
Do not use on the face or in large quantities, particularly over raw surfaces or blistered areas.
Do not use with a heating pad.
Stop use and ask a doctor if rash, itching or skin irritation develops.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of the reach of children and pets. If swallowed, get medical help or contact a Poison Control Center right away.
Package is not child-resistant.
Apply to affected area no more than three times per day.
Apply patch to the affected area and leave in place for up to 8 but no more than 12 hours.
Inactive Ingredients
Inactive ingredients
Crospovidone, menthoxypropanediol, non-woven backing fabric, polyester film, non-latex rubber based adhesive*, vanillyl butyl ether.
*adhesive not made with natural rubber latex.
Keep out of the reach of children and pets. If swallowed, get medical help or contact a Poison Control Center right away.
Package is not child-resistant.
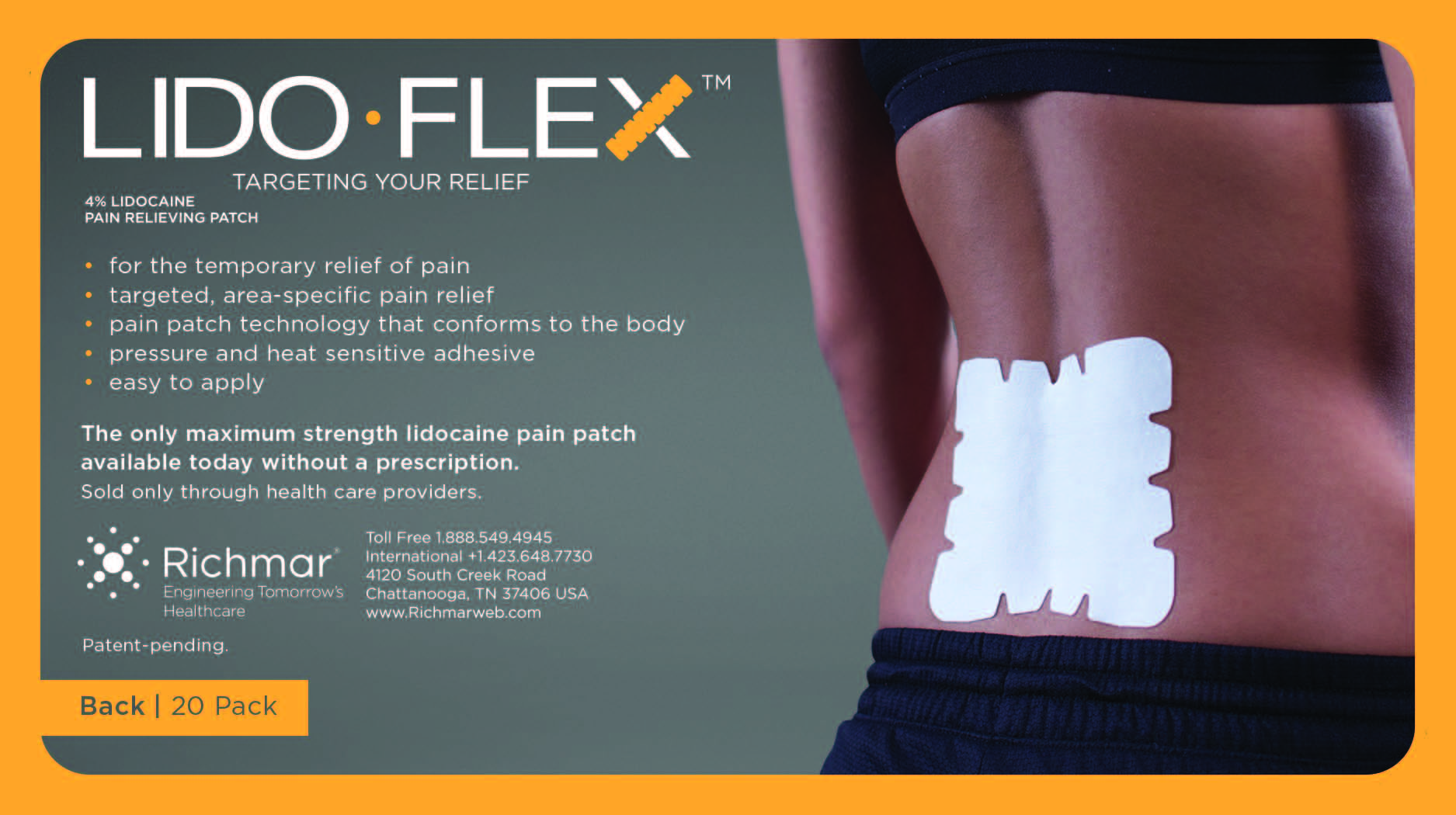
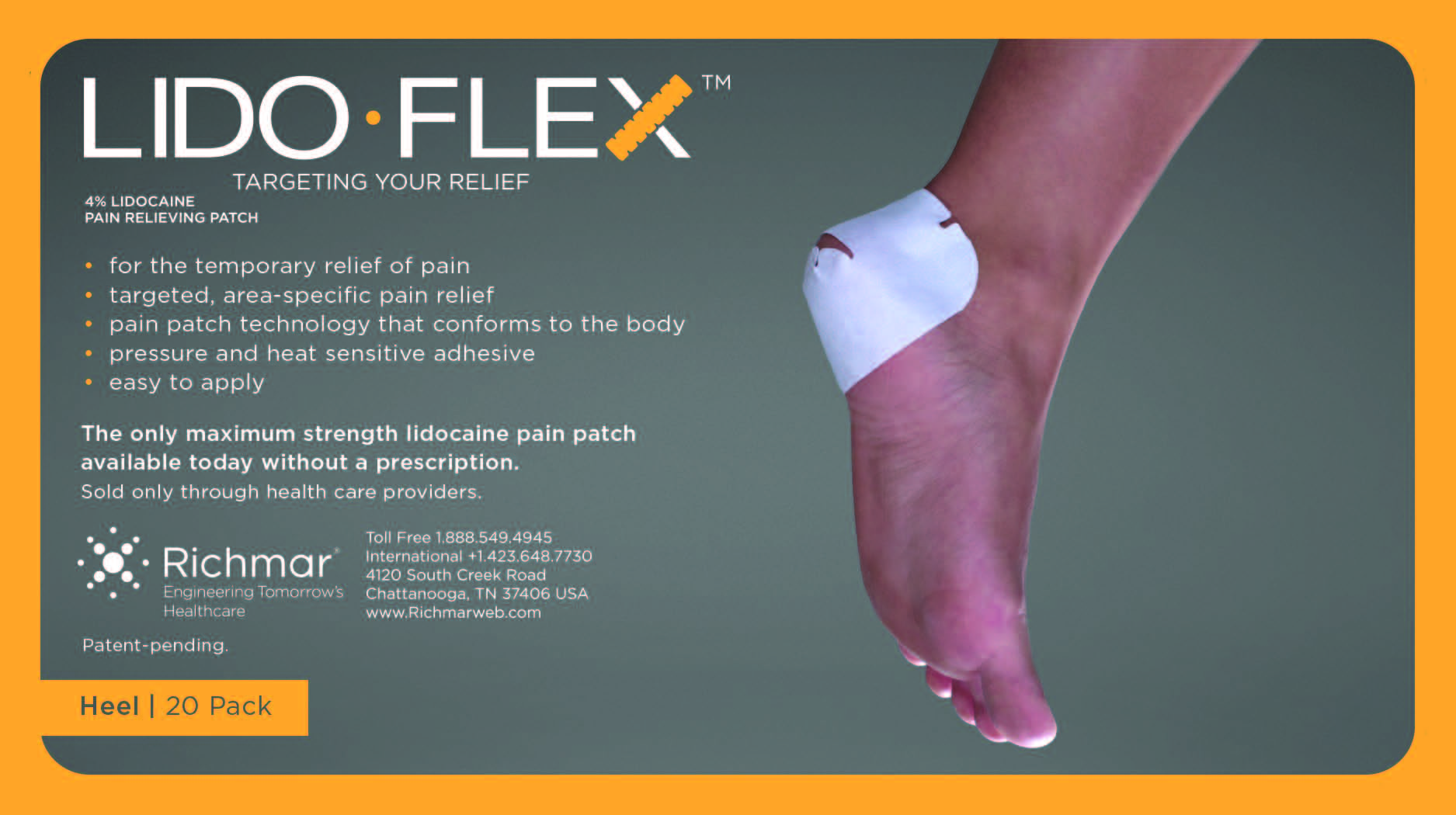
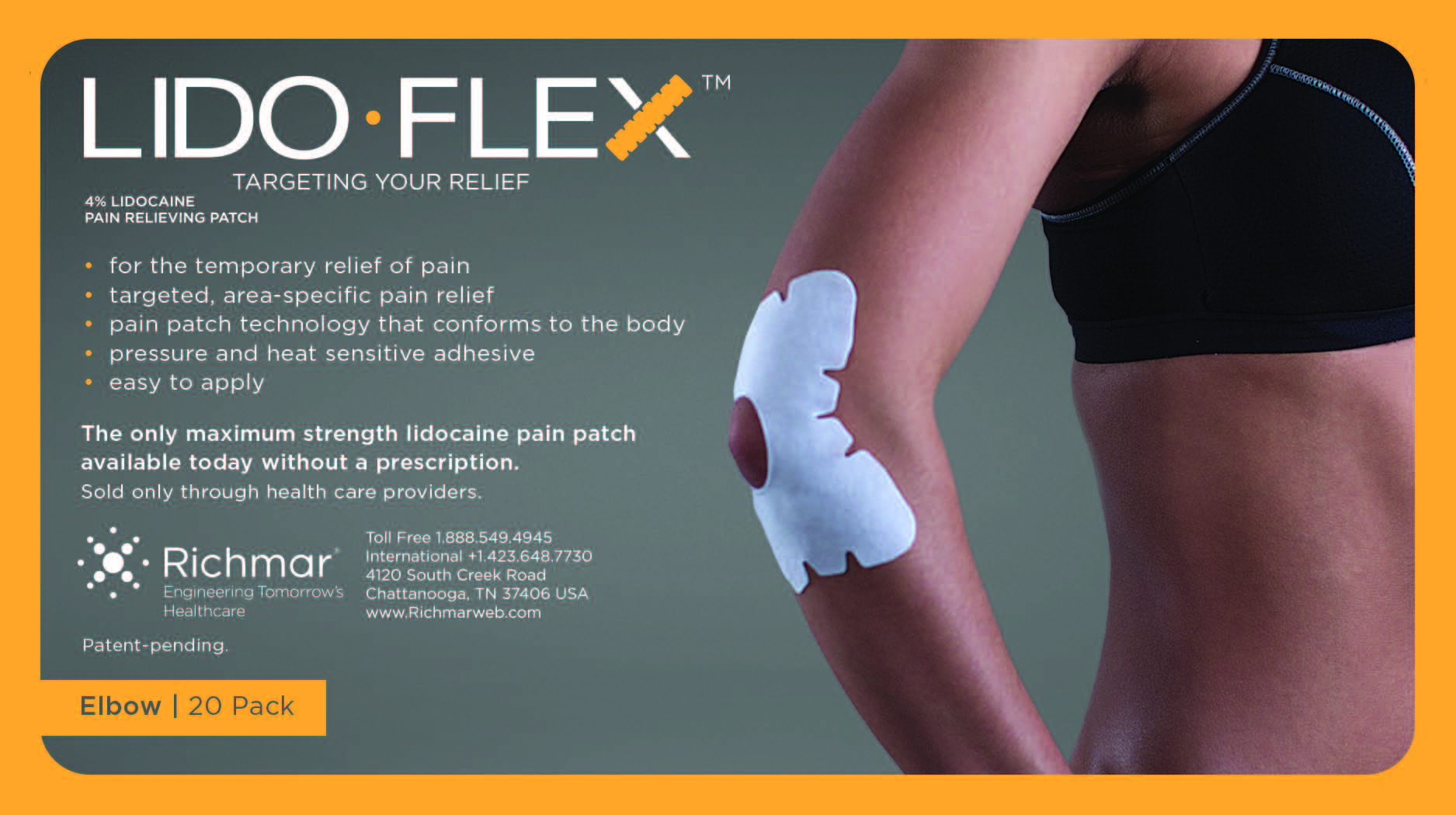
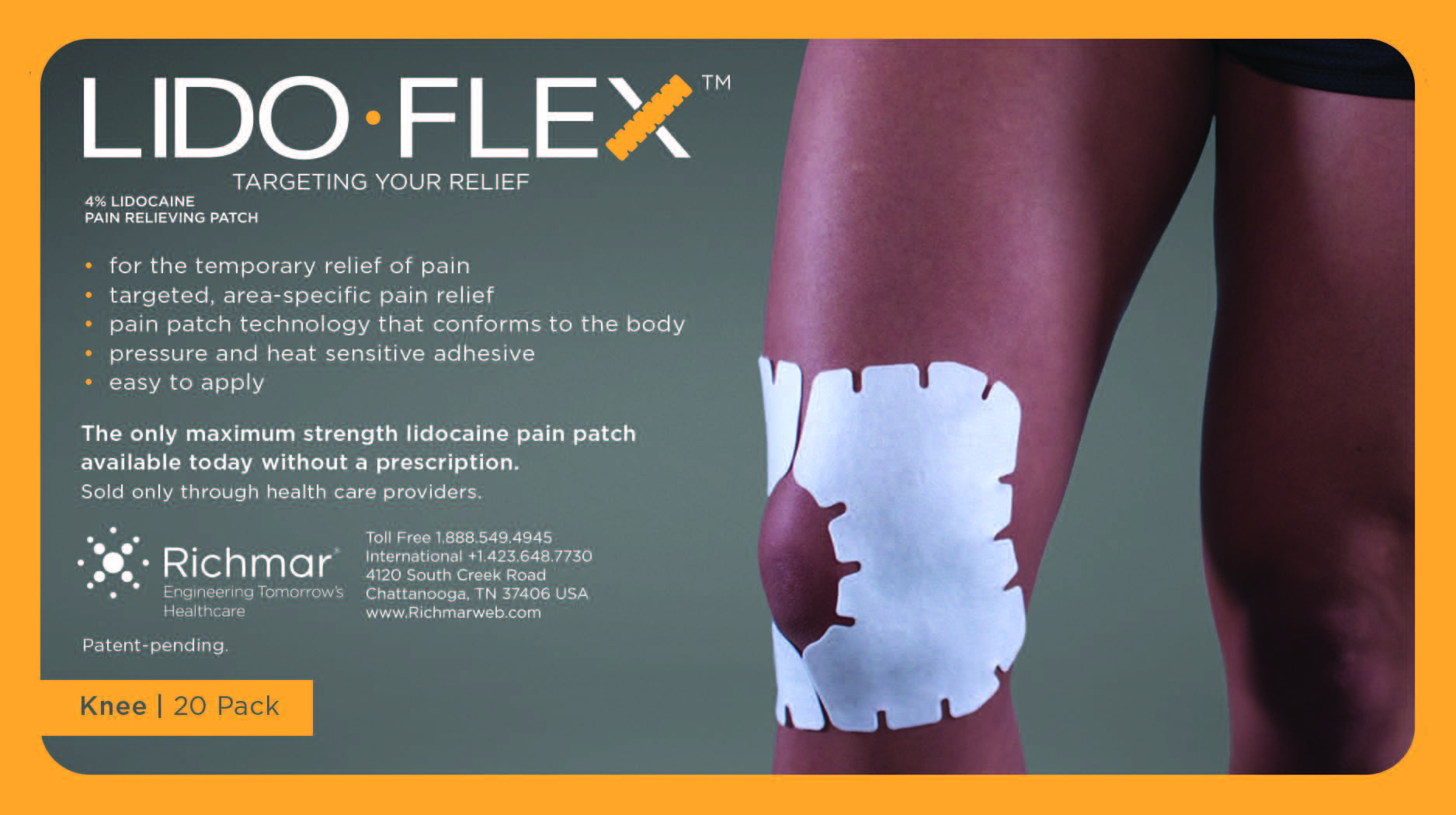
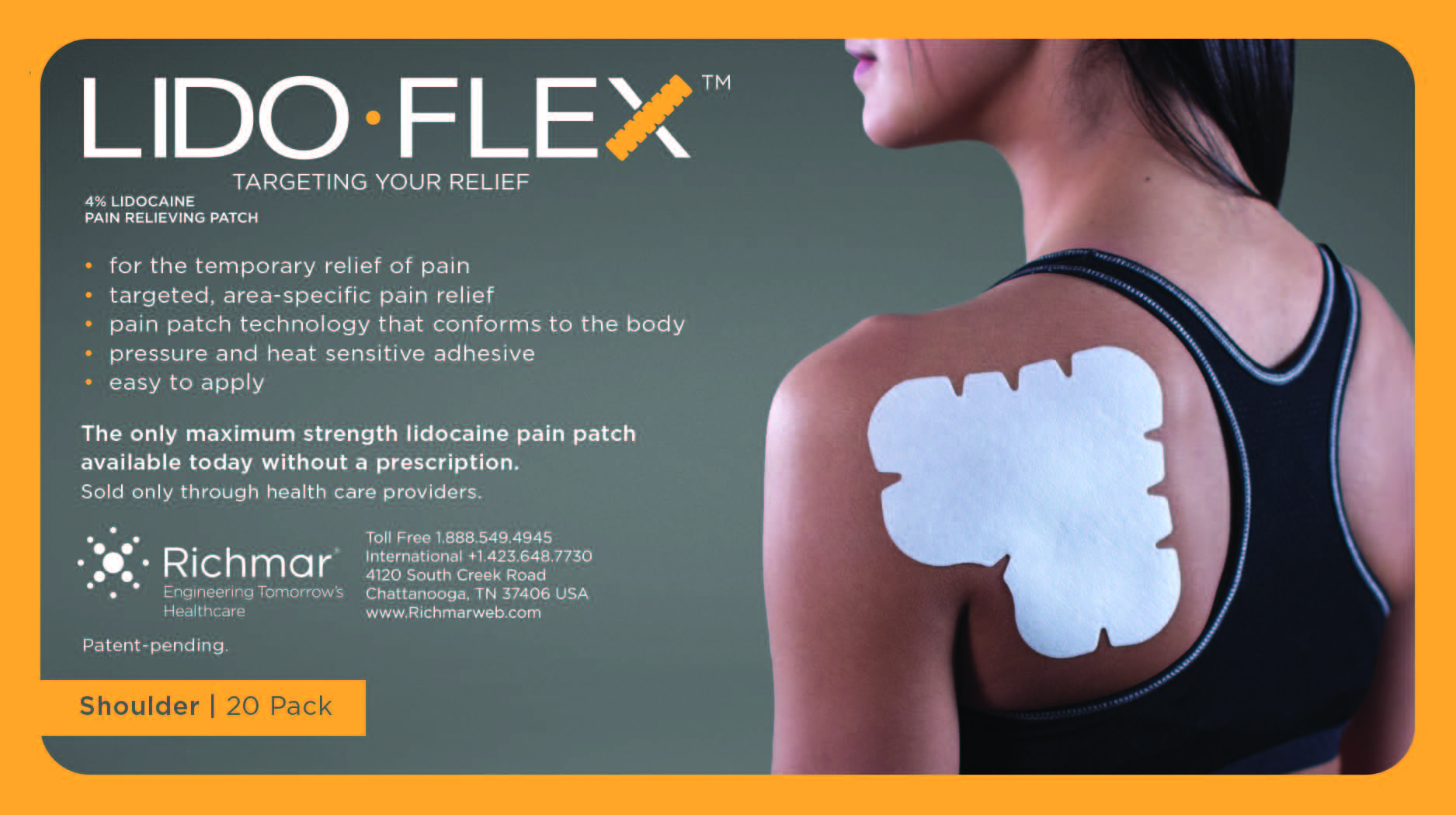
LidoFLEX 4% Lidocaine Pain Relieving Patch
 CAUTIONS
CAUTIONS
Apply to affected area no more than three (3) times per day.
Apply patch to the affected area and leave in place for up to 8 but no more than 12 hours.
DISCARDING USED PATCH
Remove the patch and fold the adhesive sides together. Discard patch safely out of the reach of
children and pets.
Patent-Pending.
4% LIDOCAINE PAIN RELIEVING PATCH
4% LIDOCANIA PARCHE PARA ALIVIAR EL DOLOR
4% LIDOCAÏNE TIMBRE DE SOULAGEMENT DE LA DOULEUR
Drug Facts
Active ingredient (in each patch) Purpose
Lidocaine 4%...........................................................Topical anesthetic/analgesic/antipruritic
Use
For the temporary relief of pain.
Warnings
Do not apply over damaged, raw or blistered skin.
Do not apply excessive quantity of patches within 24 hour period.
Apply locally as needed; do not apply over large surface area of the body.
Avoid contact with the eyes. If eye contact occurs, rinse thoroughly with water. If condition worsens, or if symptoms persist for more than 7 days
or clear up and occur again within a few days, discontinue use of this product and consult a doctor.
Do not use on the face or in large quantities, particularly over raw surfaces or blistered areas.
Do not use with a heating pad.
Stop use and ask a doctor if rash, itching or skin irritation develops.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of the reach of children and pets. If swallowed, get medical help or contact a Poison Control Center right away.
Package is not child-resistant.
Directions
Adults and children 12 years and older:
For best results:
• Remove excess hair at treatment site.
• Clean area thoroughly with soap and water or alcohol wipe, removing all oils, lotions and dirt.
• Do not use oils, lotions or powders in treatment area prior to application.
• For best adhesion, apply as directed to the affected area a minimum of 1 hour prior to getting skin wet.
• Open pouch and remove patch.
• Remove protective liner and apply to skin.
• Discard the liner.
• Apply patch to the affected area, pressing down firmly from the center outwards to ensure adhesion and minimize folds.
Continue to press down firmly until the patch has secure contact with the skin.
• Apply to affected area no more than three (3) times per day.
• Leave in place for up to 8 but no more than 12 hours.
• For maximum pain relief, choose the appropriate size of patch based on the size of the treatment area and the body area to be treated.
Example–use a LidoFlex back patch for back pain relief, use a LidoFlex Flex Strip for ankle or forearm pain relief.
Patches may be cut into smaller sizes as needed to treat affected area. Before removing the clear protective liner, cut patch to the desired size. Once cut, remove the clear protective liner. Remaining patch material must be sealed to be effective. Discard all unused portions of the patch safely out of the reach of children and pets.
Children under 12 years of age:
Ask a doctor before use.
Other information
Some individuals may not experience pain relief until several hours after applying the patch.
Store in a cool, dry place. Protect from freezing and excessive heat. Store at 68-77°F (20-25°C).
Excursions permitted between 59 and 86°F (15 and 30°C).
Use cleaning pads provided to remove any residual adhesive sticking to the skin.
Inactive ingredients
Crospovidone, menthoxypropanediol, non-woven backing fabric, polyester film, non-latex rubber based adhesive*, vanillyl butyl ether.
*adhesive not made with natural rubber latex.
Package not child-resistant. Keep out of the reach of children.
To report SUSPECTED ADVERSE REACTIONS, contact Richmar Regulatory at 1.888.549.4945 or 1.423.648.7730
www.lidoflex.com