DESCRIPTION
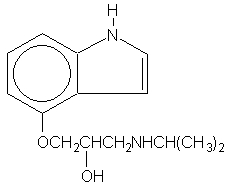
Pindolol, a synthetic beta-adrenergic receptor blocking agent with intrinsic sympathomimetic activity is 1-(Indol-4-yloxy)-3-(isopropylamino)-2-propanol.
Pindolol, USP is a white to off-white, crystalline powder having a faint odor which is practically insoluble in water; slightly soluble in methanol; and very slightly soluble in chloroform.
Each tablet for oral administration contains pindolol, USP and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, pregelatinized starch (corn), and sodium lauryl sulfate.
CLINICAL PHARMACOLOGY
Pindolol tablets are a nonselective beta-adrenergic antagonist (beta-blocker) which possesses intrinsic sympathomimetic activity (ISA) in therapeutic dosage ranges but does not possess quinidine-like membrane stabilizing activity.
PHARMACODYNAMICS
In standard pharmacologic tests in man and animals, pindolol tablets attenuate increases in heart rate, systolic blood pressure, and cardiac output resulting from exercise and isoproterenol administration, thus confirming its beta-blocking properties. The ISA or partial agonist activity of pindolol tablets is mediated directly at the adrenergic receptor sites and may be blocked by other beta-blockers. In catecholamine depleted animal experiments, ISA is manifested as an increase in the inotropic and chronotropic activity of the myocardium. In man, ISA is manifested by a smaller reduction in the resting heart rate (4 to 8 beats/min) than is seen with drugs lacking ISA. There is also a smaller reduction in resting cardiac output. The clinical significance of this observation has not been evaluated and there is no evidence, or reason to believe, that exercise cardiac output is less affected by pindolol tablets.
Pindolol tablets have been shown in controlled, double-blind clinical studies to be an effective antihypertensive agent when used as monotherapy, or when added to therapy with thiazide-type diuretics. Divided dosages in the range of 10 mg to 60 mg daily have been shown to be effective. As monotherapy, pindolol tablets are as effective as propranolol, α-methyldopa, hydrochlorothiazide, and chlorthalidone in reducing systolic and diastolic blood pressure. The effect on blood pressure is not orthostatic, i.e., pindolol tablets were equally effective in reducing the supine and standing blood pressure.
In open, long-term studies up to 4 years, no evidence of diminution of the blood pressure lowering response was observed.
An average 3-pound increase in body weight has been noted in patients treated with pindolol tablets alone, a larger increase than was observed with propranolol or placebo. The weight gain appeared unrelated to blood pressure response and was not associated with an increased risk of heart failure, although edema was more common than in control patients. Pindolol tablets do not have a consistent effect on plasma renin activity.
The mechanism of the antihypertensive effects of beta-blocking agents has not been established, but several mechanisms have been postulated: 1) an effect on the central nervous system resulting in a reduced sympathetic outflow to the periphery, 2) competitive antagonism of catecholamines at peripheral (especially cardiac) adrenergic receptor sites, leading to decreased cardiac output, 3) an inhibition of renin release. These mechanisms appear less likely for pindolol than other beta-blockers in view of the modest effect on resting cardiac output and renin.
Beta-blockade therapy is useful when it is necessary to suppress the effects of beta-adrenergic agonists in order to achieve therapeutic goals. However, in certain clinical situations, (e.g., cardiac failure, heart block, bronchospasm), the preservation of an adequate sympathetic tone may be necessary to maintain vital functions. Although a beta-antagonist with ISA such as pindolol tablets does not eliminate sympathetic tone entirely, there is no controlled evidence that it is safer than other beta-blockers in such conditions as heart failure, heart block, or bronchospasm or is less likely to cause those conditions. In single-dose studies of the effects of beta-blockers on FEV1, pindolol tablets were indistinguishable from other non-cardioselective agents in its reduction of FEV1, and its reduction in the effectiveness of an exogenous beta-agonist.
Exacerbation of angina and, in some cases, myocardial infarction and ventricular dysrhythmias have been reported after abrupt discontinuation of therapy with beta-adrenergic blocking agents in patients with coronary artery disease. Abrupt withdrawal of these agents in patients without coronary artery disease has resulted in transient symptoms, including tremulousness, sweating, palpitation, headache, and malaise. Several mechanisms have been proposed to explain these phenomena, among them increased sensitivity to catecholamines because of increased numbers of beta receptors.
PHARMACOKINETICS AND METABOLISM
Pindolol tablets are rapidly and reproducibly absorbed (greater than 95%), achieving peak plasma concentrations within 1 hour of drug administration. Pindolol tablets have no significant first-pass effect. The blood concentrations are proportional in a linear manner to the administered dose in the range of 5 mg to 20 mg. Upon repeated administration to the same subject, variation is minimal. After a single dose, intersubject variation for peak plasma concentrations was about 4-fold (e.g., 45 ng/mL to 167 ng/mL for a 20 mg dose). Upon multiple dosing, intersubject variation decreased to 2- to 2.5-fold. Pindolol tablets are only 40% bound to plasma proteins and are evenly distributed between plasma and red cells. The volume of distribution in healthy subjects is about 2 L/kg.
Pindolol tablets undergo extensive metabolism in animals and man. In man, 35% to 40% is excreted unchanged in the urine and 60% to 65% is metabolized primarily to hydroxy-metabolites which are excreted as glucuronides and ethereal sulfates. The polar metabolites are excreted with a half-life of approximately 8 hours and thus multiple dosing therapy (q.8H) results in a less than 50% accumulation in plasma. About 6% to 9% of an administered intravenous dose is excreted by the bile into the feces.
The disposition of pindolol tablets after oral administration is monophasic with a half-life in healthy subjects or hypertensive patients with normal renal function of approximately 3 to 4 hours. Following t.i.d. administration (q.8H), no significant accumulation of pindolol tablets are observed.
In elderly hypertensive patients with normal renal function, the half-life of pindolol tablets is more variable, averaging about 7 hours, but with values as high as 15 hours.
In hypertensive patients with renal diseases, the half-life is within the range expected for healthy subjects. However, a significant decrease (50%) in volume of distribution (VD) is observed in uremic patients and VD appears to be directly correlated to creatinine clearance. Therefore, renal drug clearance is significantly reduced in uremic patients, resulting in a significant decrease in urinary excretion of unchanged drug. Uremic patients with a creatinine clearance of less than 20 mL/min generally excreted less than 15% of the administered dose unchanged in the urine.
In patients with histologically diagnosed cirrhosis of the liver, the elimination of pindolol tablets was more variable in rate and generally significantly slower than in healthy subjects. The total body clearance of pindolol tablets in cirrhotic patients ranged from about 50 mL/min to 300 mL/min and was directly correlated to antipyrine clearance. The half-life ranges from 2.5 hours to greater than 30 hours. These findings strongly suggest that caution should be exercised in dosage adjustments of pindolol tablets in such patients.
The bioavailability of pindolol tablets is not significantly affected by coadministration of food, hydralazine, hydrochlorothiazide or aspirin. Pindolol tablets have no effect on warfarin activity or the clinical effectiveness of digoxin, although small transient decreases in plasma digoxin concentrations were noted.
INDICATIONS AND USAGE
Pindolol tablets are indicated in the management of hypertension. It may be used alone or concomitantly with other antihypertensive agents, particularly with a thiazide-type diuretic.
CONTRAINDICATIONS
Pindolol tablets are contraindicated in: 1) bronchial asthma; 2) overt cardiac failure; 3) cardiogenic shock; 4) second and third degree heart block; 5) severe bradycardia. (See WARNINGS.)
WARNINGS
Cardiac Failure
Sympathetic stimulation may be a vital component supporting circulatory function in patients with congestive heart failure, and its inhibition by beta-blockade may precipitate more severe failure. Although beta-blockers should be avoided in overt congestive heart failure, if necessary, pindolol tablets can be used with caution in patients with a history of failure who are well-compensated, usually with digitalis and diuretics. Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase risk of bradycardia. Beta-adrenergic blocking agents do not abolish the inotropic action of digitalis on heart muscle.
In Patients Without A History of Cardiac Failure
In patients with latent cardiac insufficiency, continued depression of the myocardium with beta-blocking agents over a period of time can in some cases lead to cardiac failure. At the first sign or symptom of impending cardiac failure, patients should be fully digitalized and/or be given a diuretic, and the response observed closely. If cardiac failure continues, despite adequate digitalization and diuretic, pindolol tablets therapy should be withdrawn (gradually, if possible).
Exacerbation of Ischemic Heart Disease Following Abrupt Withdrawal
Hypersensitivity to catecholamines has been observed in patients withdrawn from beta-blocker therapy; exacerbation of angina and, in some cases, myocardial infarction have occurred after abrupt discontinuation of such therapy. When discontinuing chronically administered pindolol tablets, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of 1 to 2 weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, pindolol tablets administration should be reinstituted promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Patients should be warned against interruption or discontinuation of therapy without the physician’s advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue pindolol tablets therapy abruptly even in patients treated only for hypertension.
Nonallergic Bronchospasm (e.g., chronic bronchitis, emphysema) - Patients with Bronchospastic Diseases Should in General Not Receive Beta-Blockers
Pindolol tablets should be administered with caution since it may block bronchodilation produced by endogenous or exogenous catecholamine stimulation of beta2 receptors.
Major Surgery
Because beta-blockade impairs the ability of the heart to respond to reflex stimuli and may increase the risks of general anesthesia and surgical procedures, resulting in protracted hypotension or low cardiac output, it has generally been suggested that such therapy should be gradually withdrawn several days prior to surgery. Recognition of the increased sensitivity to catecholamines of patients recently withdrawn from beta-blocker therapy, however, has made this recommendation controversial. If possible, beta-blockers should be withdrawn well before surgery takes place. In the event of emergency surgery, the anesthesiologist should be informed that the patient is on beta-blocker therapy.
The effects of pindolol tablets can be reversed by administration of beta-receptor agonists such as isoproterenol, dopamine, dobutamine, or norepinephrine. Difficulty in restarting and maintaining the heart beat has also been reported with beta-adrenergic receptor blocking agents.
Diabetes and Hypoglycemia
Beta-adrenergic blockade may prevent the appearance of premonitory signs and symptoms (e.g., tachycardia and blood pressure changes) of acute hypoglycemia. This is especially important with labile diabetics. Beta-blockade also reduces the release of insulin in response to hyperglycemia; therefore, it may be necessary to adjust the dose of antidiabetic drugs.
PRECAUTIONS
Impaired Renal or Hepatic Function
Beta-blocking agents should be used with caution in patients with impaired hepatic or renal function. Poor renal function has only minor effects on pindolol tablets clearance, but poor hepatic function may cause blood levels of pindolol to increase substantially.
Information for Patients
Patients, especially those with evidence of coronary artery insufficiency, should be warned against interruption or discontinuation of pindolol tablets therapy without the physician’s advice. Although cardiac failure rarely occurs in properly selected patients, patients being treated with beta-adrenergic blocking agents should be advised to consult the physician at the first sign or symptom of impending failure.
Drug Interactions
Catecholamine-depleting drugs (e.g., reserpine) may have an additive effect when given with beta-blocking agents. Patients receiving pindolol tablets plus a catecholamine-depleting agent should, therefore, be closely observed for evidence of hypotension and/or marked bradycardia which may produce vertigo, syncope, or postural hypotension.
Pindolol tablets have been used with a variety of antihypertensive agents, including hydrochlorothiazide, hydralazine, and guanethidine without unexpected adverse interactions.
Pindolol tablets have been shown to increase serum thioridazine levels when both drugs are coadministered. Pindolol levels may also be increased with this combination.
Risk of Anaphylactic Reaction
While taking beta-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reactions.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In chronic oral toxicologic studies (1 to 2 years) in mice, rats, and dogs, pindolol tablets did not produce any significant toxic effects. In 2-year oral carcinogenicity studies in rats and mice in doses as high as 59 mg/kg/day and 124 mg/kg/day (50 and 100 times the maximum recommended human dose), respectively, pindolol tablets did not produce any neoplastic, preneoplastic, or nonneoplastic pathologic lesions. In fertility and general reproductive performance studies in rats, pindolol tablets caused no adverse effects at a dose of 10 mg/kg.
In the male fertility and general reproductive performance test in rats, definite toxicity characterized by mortality and decreased weight gain was observed in the group given 100 mg/kg/day. At 30 mg/kg/day, decreased mating was associated with testicular atrophy and/or decreased spermatogenesis. This response is not clearly drug-related, however, as there was no dose-response relationship within this experiment and no similar effect on testes of rats administered pindolol tablets as a dietary admixture for 104 weeks. There appeared to be an increase in prenatal mortality in males given 100 mg/kg but development of offspring was not impaired.
In females administered pindolol tablets prior to mating through day 21 of lactation, mating behavior was decreased at 100 mg/kg and 30 mg/kg. At these dosages there also was increased mortality of offspring. Prenatal mortality was increased at 10 mg/kg but there was not a clear dose-response relationship in this experiment. There was an increased resorption rate at 100 mg/kg observed in females necropsied on the 15th day of gestation.
Pregnancy
Teratogenic Effects. Category B
Studies in rats and rabbits exceeding 100 times the maximum recommended human doses, revealed no embryotoxicity or teratogenicity. Since there are no adequate and well controlled studies in pregnant women, and since animal reproduction studies are not always predictive of human response, pindolol tablets, as with any drug, should be employed during pregnancy only if the potential benefit justifies the potential risk to the fetus.
CLINICAL LABORATORY
Minor persistent elevations in serum transaminases (SGOT, SGPT) have been noted in 7% of patients during pindolol tablets administration, but progressive elevations were not observed. These elevations were not associated with any other abnormalities that would suggest hepatic impairment, such as decreased serum albumin and total proteins. During more than a decade of worldwide marketing, there have been no reports in the medical literature of overt hepatic injury. Alkaline phosphatase, lactic acid dehydrogenase (LDH), and uric acid are also elevated on rare occasions. The significance of these findings is unknown.
ADVERSE REACTIONS
Most adverse reactions have been mild. The incidences listed in the following table are derived from 12-week comparative double-blind, parallel design trials in hypertensive patients given pindolol tablets as monotherapy, given various active control drugs as monotherapy, or given placebo. Data for pindolol tablets and the positive controls were pooled from several trials because no striking differences were seen in the individual studies, with one exception. When considering all adverse reactions reported, the frequency of edema was noticeably higher in positive control trials (16% pindolol tablets vs. 9% positive control) than in placebo controlled trials (6% pindolol tablets vs. 3% placebo). The table includes adverse reactions either volunteered or elicited, and at least possibly drug-related, which were reported in greater than 2% of pindolol tablets patients and other selected important reactions.
|
|||
|
(and at least possibly drug-related) |
|||
|
Body System/
|
Pindolol Tablets
|
Active Controls*
|
Placebo
|
|
Central Nervous System | |||
|
Bizarre or Many Dreams |
5 |
0 |
6 |
|
Dizziness |
9 |
11 |
1 |
|
Fatigue |
8 |
4 |
4 |
|
Hallucinations |
< 1 |
0 |
0 |
|
Insomnia |
10 |
3 |
10 |
|
Nervousness |
7 |
3 |
5 |
|
Weakness |
4 |
2 |
1 |
|
Autonomic Nervous System | |||
|
Paresthesia |
3 |
1 |
6 |
|
Cardiovascular | |||
|
Dyspnea |
5 |
4 |
6 |
|
Edema |
6 |
3 |
1 |
|
Heart Failure |
< 1 |
< 1 |
0 |
|
Palpitations |
< 1 |
1 |
0 |
|
Musculoskeletal | |||
|
Chest Pain |
3 |
1 |
3 |
|
Joint Pain |
7 |
4 |
4 |
|
Muscle Cramps |
3 |
1 |
0 |
|
Muscle Pain |
10 |
9 |
8 |
|
Gastrointestinal | |||
|
Abdominal Discomfort |
4 |
4 |
5 |
|
Nausea |
5 |
2 |
1 |
|
Skin | |||
|
Pruritus |
1 |
< 1 |
0 |
|
Rash |
< 1 |
< 1 |
1 |
The following selected (potentially important) adverse reactions were seen in 2% or fewer patients and their relationship to pindolol tablets is uncertain. CENTRAL NERVOUS SYSTEM: anxiety, lethargy; AUTONOMIC NERVOUS SYSTEM: visual disturbances, hyperhidrosis; CARDIOVASCULAR: bradycardia, claudication, cold extremities, heart block, hypotension, syncope, tachycardia, weight gain; GASTROINTESTINAL: diarrhea, vomiting; RESPIRATORY: wheezing; UROGENITAL: impotence, pollakiuria; MISCELLANEOUS: eye discomfort or burning eyes.
POTENTIAL ADVERSE EFFECTS
In addition, other adverse effects not aforementioned have been reported with other beta-adrenergic blocking agents and should be considered potential adverse effects of pindolol tablets.
Central Nervous System: Reversible mental depression progressing to catatonia; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance on neuropsychometrics.
Cardiovascular: Intensification of AV block. (See CONTRAINDICATIONS.)
Allergic: Erythematous rash; fever combined with aching and sore throat; laryngospasm; respiratory distress.
Hematologic: Agranulocytosis; thrombocytopenic and nonthrombocytopenic purpura.
Gastrointestinal: Mesenteric arterial thrombosis; ischemic colitis.
Miscellaneous: Reversible alopecia; Peyronie’s disease.
The oculomucocutaneous syndrome associated with the beta-blocker practolol has not been reported with pindolol tablets during investigational use and extensive foreign experience amounting to over 4 million patient-years.
OVERDOSAGE
No specific information on emergency treatment of overdosage is available. Therefore, on the basis of the pharmacologic actions of pindolol tablets, the following general measures should be employed as appropriate in addition to gastric lavage:
Excessive Bradycardia: administer atropine; if there is no response to vagal blockade, administer isoproterenol cautiously.
Cardiac Failure: digitalize the patient and/or administer diuretic. It has been reported that glucagon may be useful in this situation.
Hypotension: administer vasopressors, e.g., epinephrine or norepinephrine, with serial monitoring of blood pressure. (There is evidence that epinephrine may be the drug of choice.)
Bronchospasm: administer a beta2 stimulating agent such as isoproterenol and/or a theophylline derivative.
A case of an acute overdosage has been reported with an intake of 500 mg of pindolol tablets by a hypertensive patient. Blood pressure increased and heart rate was ≥ 80 beats/min. Recovery was uneventful. In another case, 250 mg of pindolol tablets was taken with 150 mg diazepam and 50 mg nitrazepam, producing coma and hypotension. The patient recovered in 24 hours.
DOSAGE AND ADMINISTRATION
The dosage of pindolol tablets should be individualized. The recommended initial dose of pindolol tablets is 5 mg b.i.d. alone or in combination with other antihypertensive agents. An antihypertensive response usually occurs within the first week of treatment. Maximal response, however, may take as long as or occasionally longer than 2 weeks. If a satisfactory reduction in blood pressure does not occur within 3 to 4 weeks, the dose may be adjusted in increments of 10 mg/day at these intervals up to a maximum of 60 mg/day.
HOW SUPPLIED
Pindolol Tablets, USP are available containing 5 mg or 10 mg of pindolol, USP.
The 5 mg tablets are white, round, scored tablets debossed with M above the score and 52 below the score on one side of the tablet and blank on the other side. They are available as follows:
NDC 0378-0052-01
bottles of 100 tablets
The 10 mg tablets are white, round, scored tablets debossed with M above the score and 127 below the score on one side of the tablet and blank on the other side. They are available as follows:
NDC 0378-0127-01
bottles of 100 tablets
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A
Revised: 11/2016
PIND:R9
PRINCIPAL DISPLAY PANEL - 5 mg
NDC 0378-0052-01
Pindolol
Tablets, USP
5 mg
Rx only 100 Tablets
Each tablet contains:
Pindolol, USP 5 mg
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Keep this and all medication
out of the reach of children.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]
Protect from light.
Usual Adult Dosage: One tablet
twice daily. See accompanying
prescribing information.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Mylan.com
RM0052A6
PRINCIPAL DISPLAY PANEL - 10 mg
NDC 0378-0127-01
Pindolol
Tablets, USP
10 mg
Rx only 100 Tablets
Each tablet contains:
Pindolol, USP 10 mg
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Keep this and all medication
out of the reach of children.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]
Protect from light.
Usual Adult Dosage: One tablet
twice daily. See accompanying
prescribing information.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Mylan.com
RM0127A6