Active ingredients
Each tablet contains:
Branhamella catarrhalis 200K
Influenzinum 200K
Klebsiella pneumoniae 200K
Micrococcus tetragenus 200K
Warnings
This product is not intended to be an alternative to
vaccination.
Do not use if you have ever had an allergic reaction to this
product or any of its ingredients.
Stop use and ask a doctor if symptoms continue to persist
after three days of use or are accompanied by a fever.
If pregnant or breastfeeding, ask a health professional before
use. Keep out of reach of children. In case of overdose, get
medical help or contact a Poison Control Center right away.
Inactive ingredients
Carboxymethylcellulose sodium, lactose monohydrate (milk), magnesium stearate, xylitol
Other information
Do not use if the blister pack has been tampered with.
Store in a cool, dry place.
Directions
Adults and Adolescents (12 years and older): At the first sign of symptoms, take one tablet allowing it to dissolve under the tongue. If
symptoms persist, take one tablet two or three times during the day or as recommended by your healthcare practitioner.
Children (6-11 years): Take under the direction of your healthcare practitioner.
Uses
For the temporary relief of cold and flu-like symptoms.
Directions
Adults and Adolescents (12 years and older): At the first sign of symptoms, take one tablet allowing it to dissolve under the tongue. If
symptoms persist, take one tablet two or three times during the day or as recommended by your healthcare practitioner.
Children (6-11 years): Take under the direction of your healthcare practitioner.
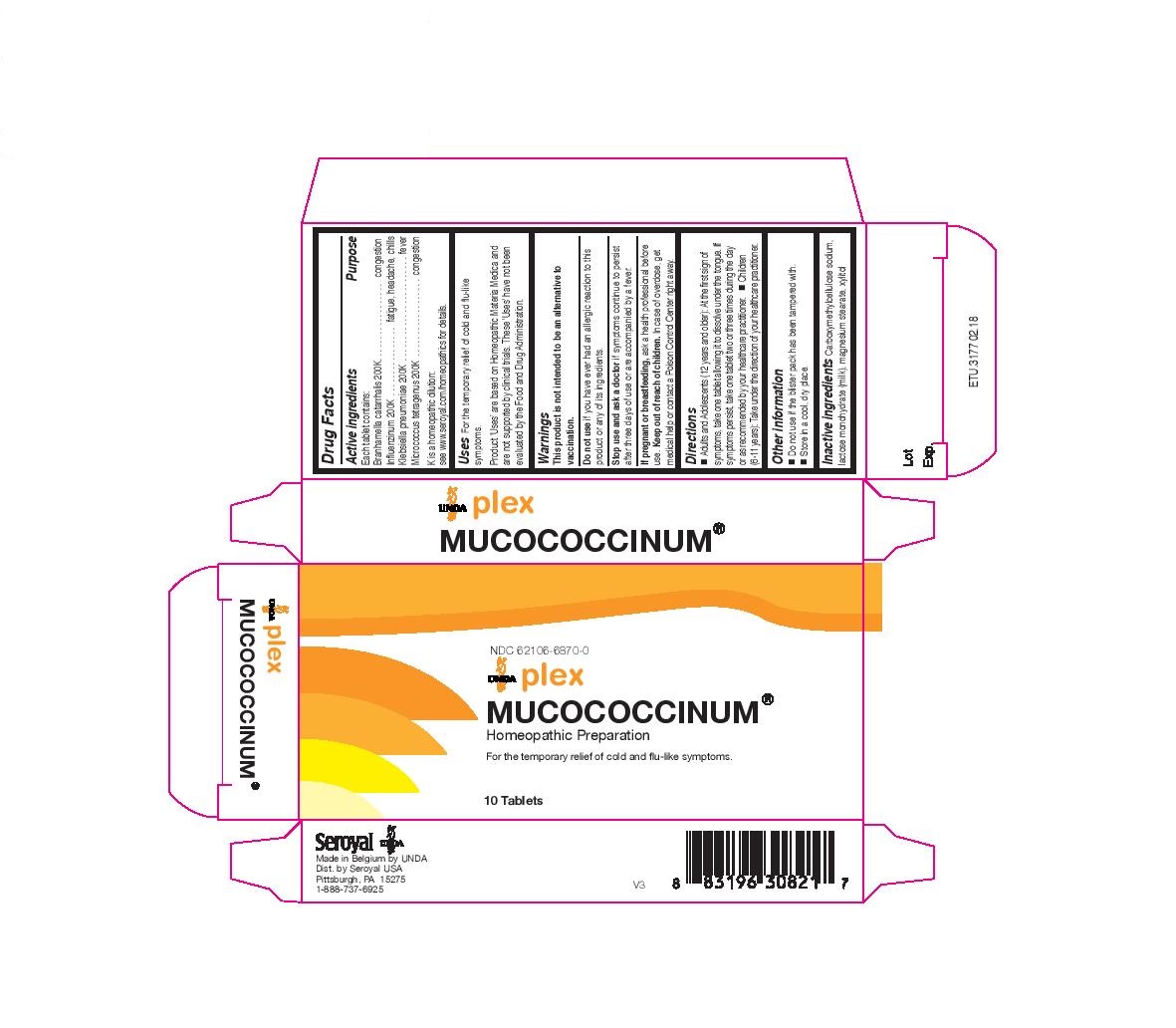 NDC 62106-6870-0
NDC 62106-6870-0