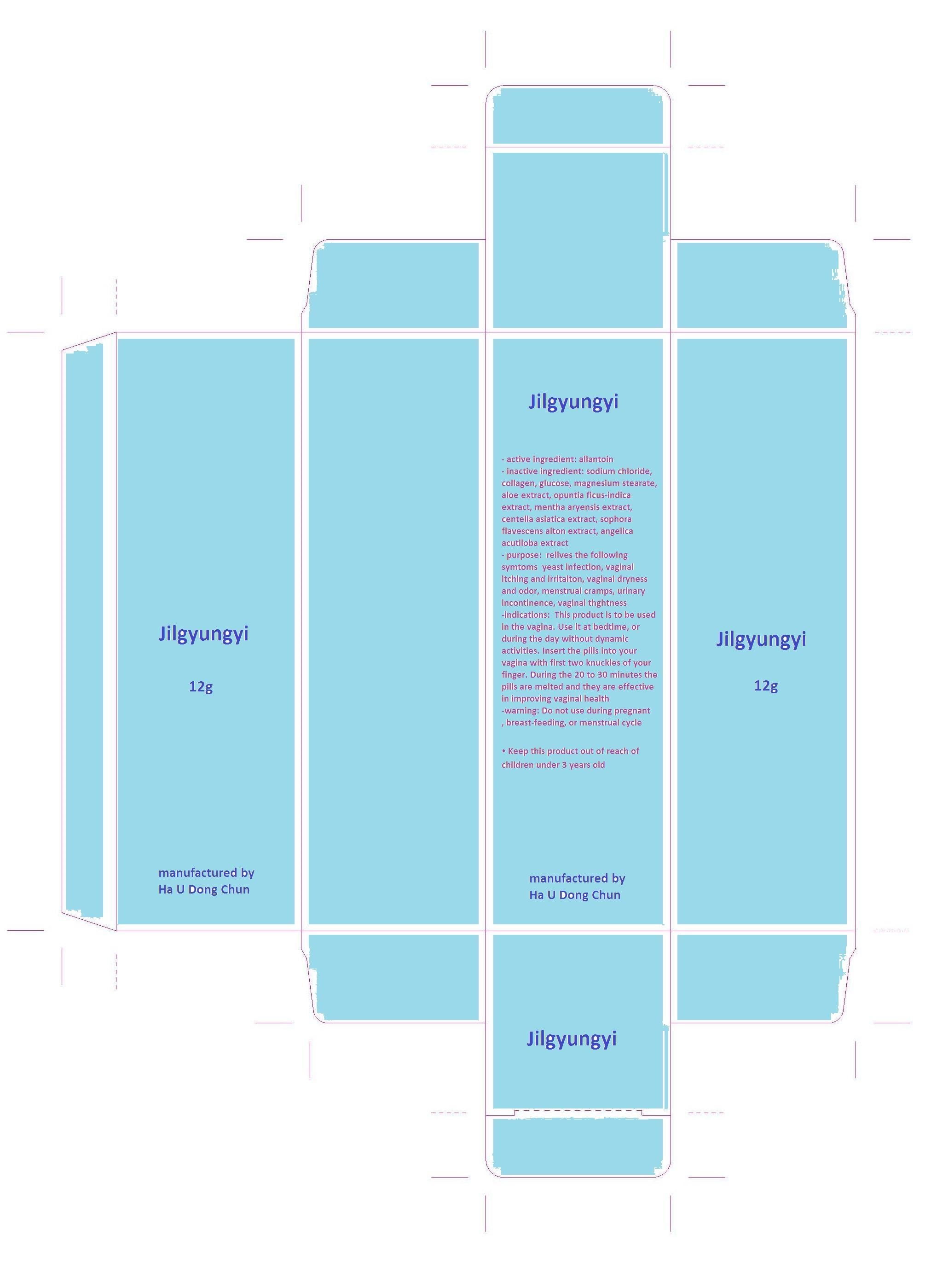
sodium chloride, collagen, glucose, magnesium stearate, aloe extract, opuntia ficus-indica extract, mentha aryensis extract, centella asiatica extract, sophora flavescens aiton extract, angelica acutiloba extract
- relives the following symtoms
yeast infection, vaginal itching and irritaiton, vaginal dryness and odor, menstrual cramps, urinary incontinence, vaginal thghtness
This product is to be used in the vagina. Use it at bedtime, or during the day without dynamic activities. Insert the pills into your vagina with first two knuckles of your finger. During the 20 to 30 minutes the pills are melted and they are effective in improving vaginal health