Take one tablet orally as directed by your physician as needed for symptoms of panic or anxiety.
Do not exceed two tablets per day.
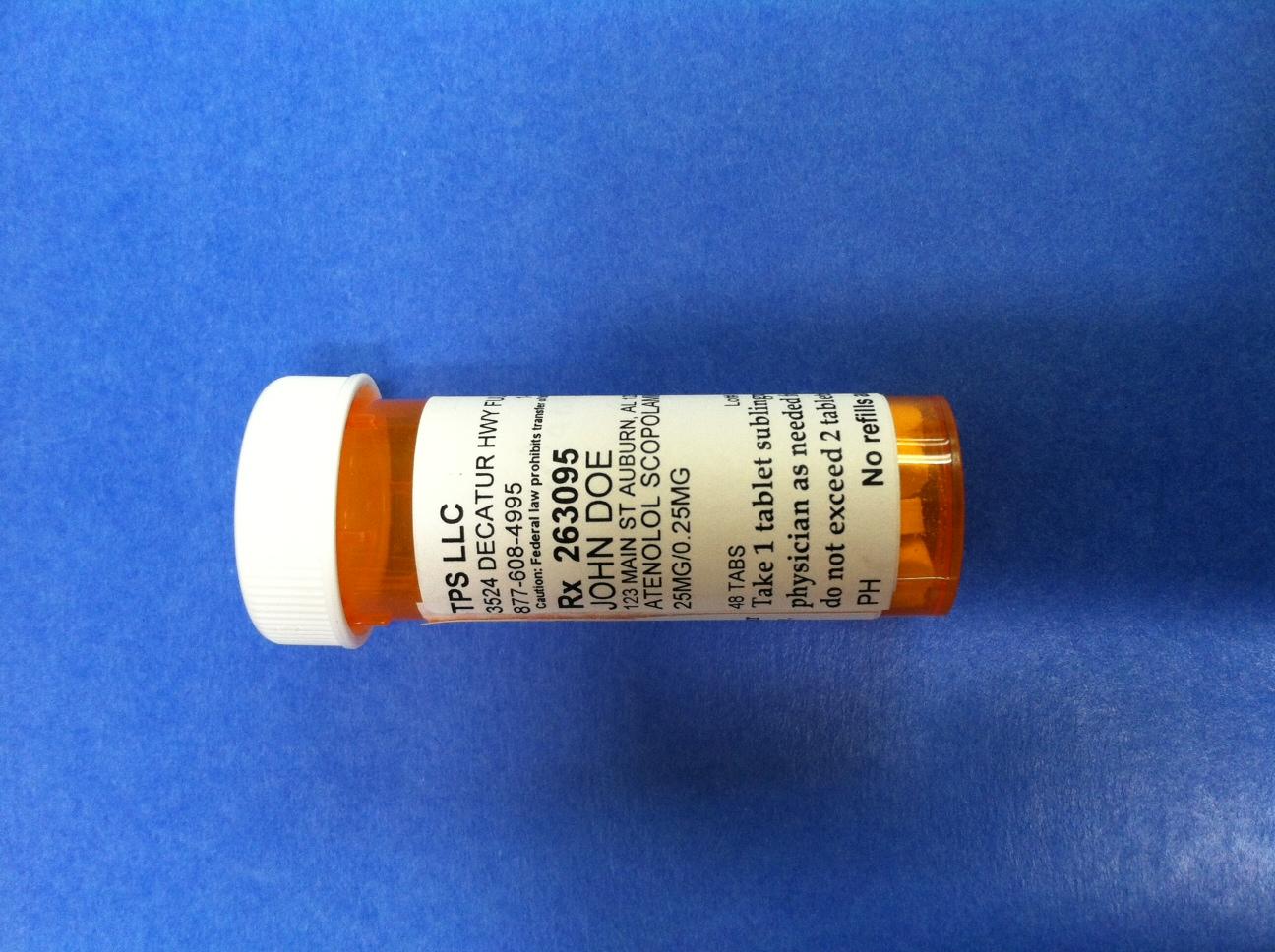 TPS LLC
TPS LLC
3524 DECATUR HWY FULTONDALE, AL 35068
877-608-4995 1-877-608-4995 BT9752747
Caution: Federal law prohibits transfer of this drug to any other person than patient for whom prescribed
Rx 263095 Jack Doe/Dr. Jane Doe MD
JOHN DOE
123 MAIN ST AUBURN, AL 12345
PROPRANOLOL SCOPOLAMINE TABLET TRITURATE
20 MG/0.25 MG
48 TABS Lot# Exp
Take 1 tablet sublingually or orally as directed by your physician as needed for symptoms of panic or anxiety.
Do not exceed 2 tablets per day
PH No refills authorized 10/8/2014
Pill bottle low res.jpg