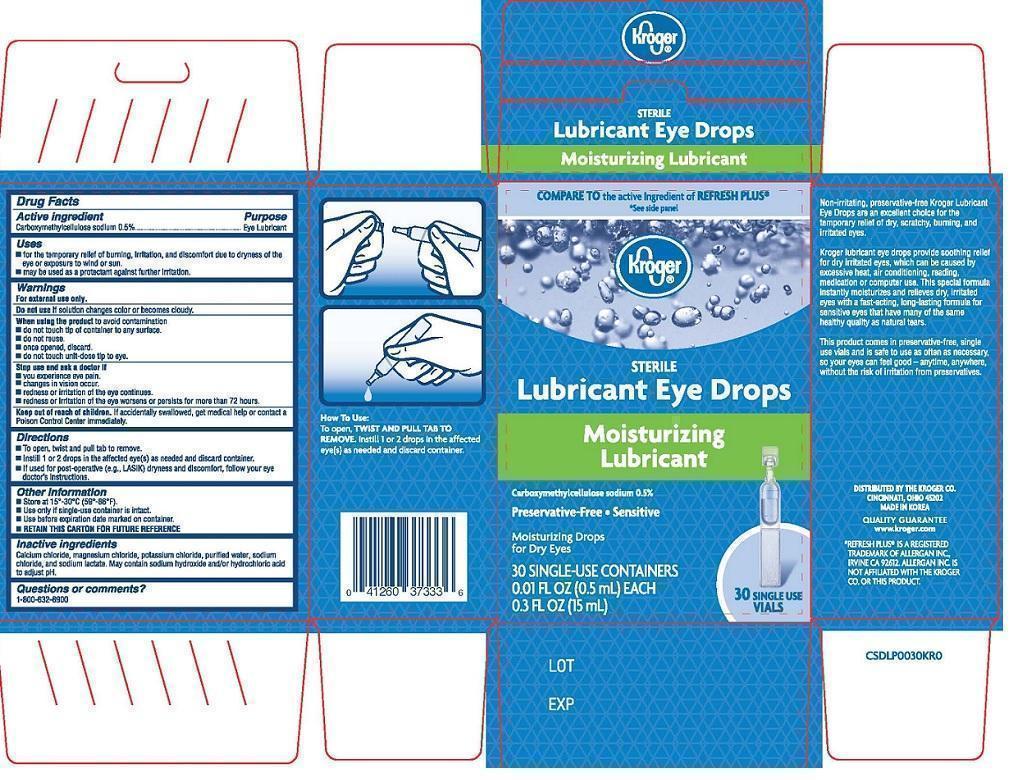
Uses
- for the temporary relief of burning, irritation, and discomfort due to dryness of the eye or exposure to wind or sun
- may be used as a protectant against further irritation
Warnings
For external use only.
When using the product to avoid contamination
- do not touch tip of container to any surface.
- do not reuse.
- once opened, discard.
- do not touch unit-dose tip to eye.
Directions
- To open, twist and pull tab to remove.
- Instill 1 or 2 drops in the affected eye(s) as needed and discard container.
- If used for post-operative (e.g., LASIK) dryness and discomfort, follow your eye doctor’s instructions.
Other information
- Store at 15°-30°C (59°-86°F).
- Use only if single-use container is intact.
- Use before expiration date marked on container.
- RETAIN THIS CARTON FOR FUTURE REFERENCE