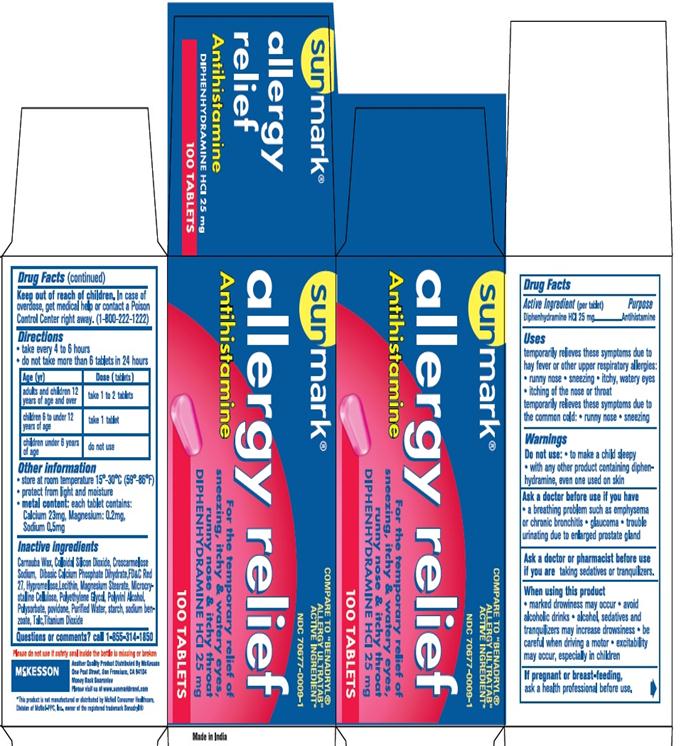
Uses
temporarily relieves these symptoms of hay fever or other upper respiratory allergies:
- runny nose
- itching of the nose or throat
- sneezing
- itchy, watery eyes
temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
When using this product
- you may get very drowsy
- avoid alcoholic drinks
- alcohol, sedatives & tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
- take every 4 to 6 hours
- do not take more than 6 tablets in 24 hours
Age (Yr) Dose (tablets) Adults and children 12 years of age and over Take 1 to 2 tablets Children 6 to under 12 years of age Take 1 tablet Children under 6 years of age do not use
Other Information
- store at 25°C(77 °F) excusrsions permitted between 15°-30°C (59°-86°F)
- protect from light and moisture
- metal content: each tablet contains: Calcium 23mg, Magnesium: 0.2 mg, sodium 0.5 mg
Inactive Ingredients
Carnauba Wax, Colloidal silion dioxide, croscarmellose sodium, D&C red #27 al lake, dibasic calcium phosphate dihydrate, hypromellose, Lecithin, magnesium stearate, microcrystalline cellulose, polyethylene glycol, Polyvinyl Alcohol, polysorbate, Povidone, Purified water, starch, sodium benozoate, talc,Titanium Dioxide