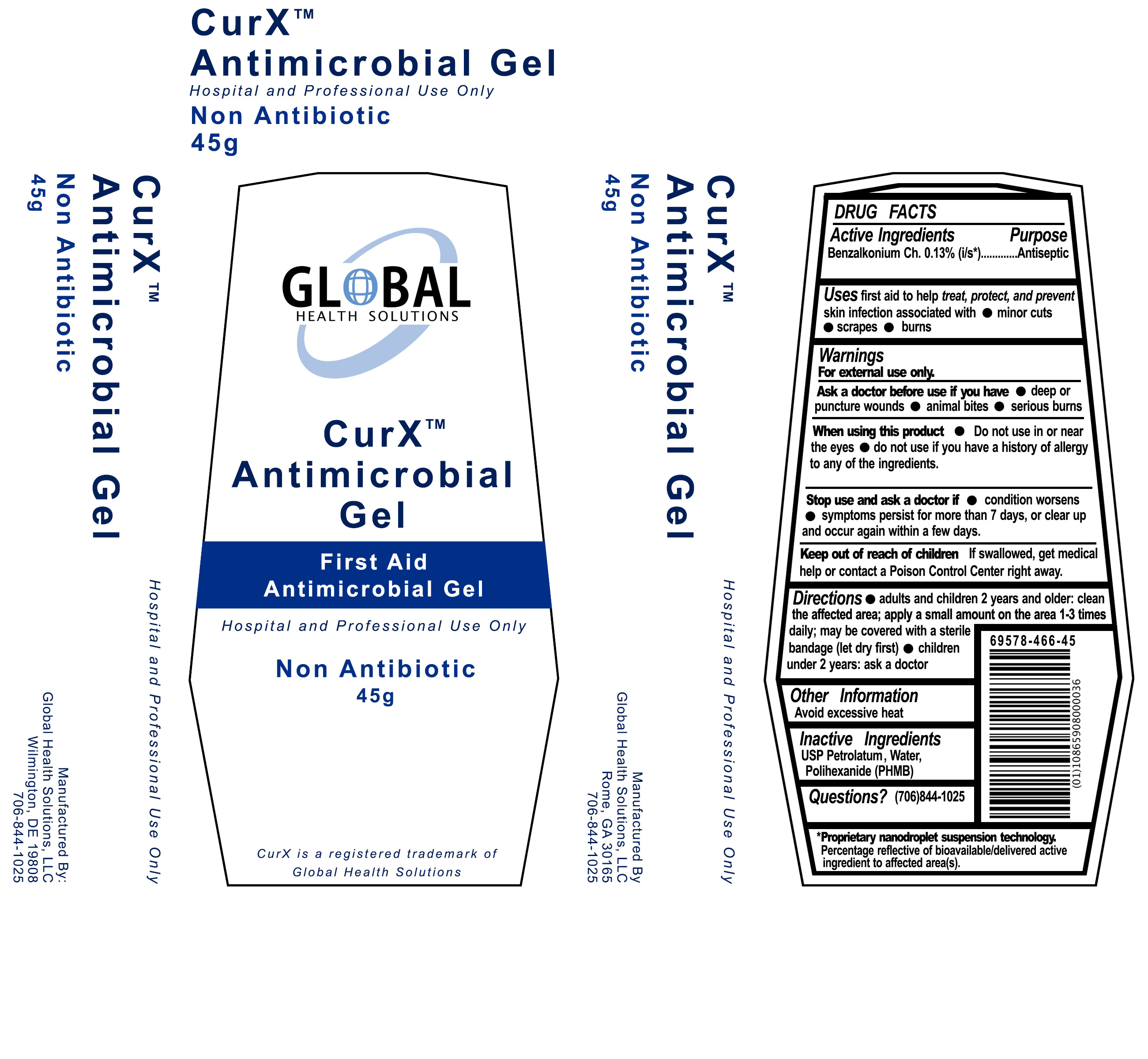
CURX ANTIMICROBIAL GEL - benzalkonium chloride gel
Global Health Solutions, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients
Benzalkonium Ch. 0.13% (i/s*)
Purpose
First Aid Antiseptic
Uses
first aid to to help treat, protect and prevent skin infection associated with
Warnings
For external use only
Ask a doctor before use if you have
When using this product
- Do not use in or near the eyes
- do not use if you have a history of allergy to any of the ingredients.
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days, or clear up and occur again within a few days.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 years and older: clean the affected area; apply a small amount on the area 1-3 times daily;may be covered with a sterile bandage (let dry first)
- children under 2 years: ask a doctor
Other Information
Avoid excessive heat
Inactive Ingredients
USP Petrolatum, Water, Polihexanide (PHMB)
Questions?
1-706-844-1025
Principal Display Panel
GLOBAL HEALTH SOLUTIONS, LLC
CurXTM Antimicrobial Gel
First Aid Antimicrobial Gel
Hospital and Professional Use Only
Non-Antibiotic
45 g
CurX is a registered trademark of Global Health Solutions
*Microdroplet Suspension Technology
*Proprietary microdroplet suspension technology. Percentage reflective of active ingredient bioavailability in solution.
