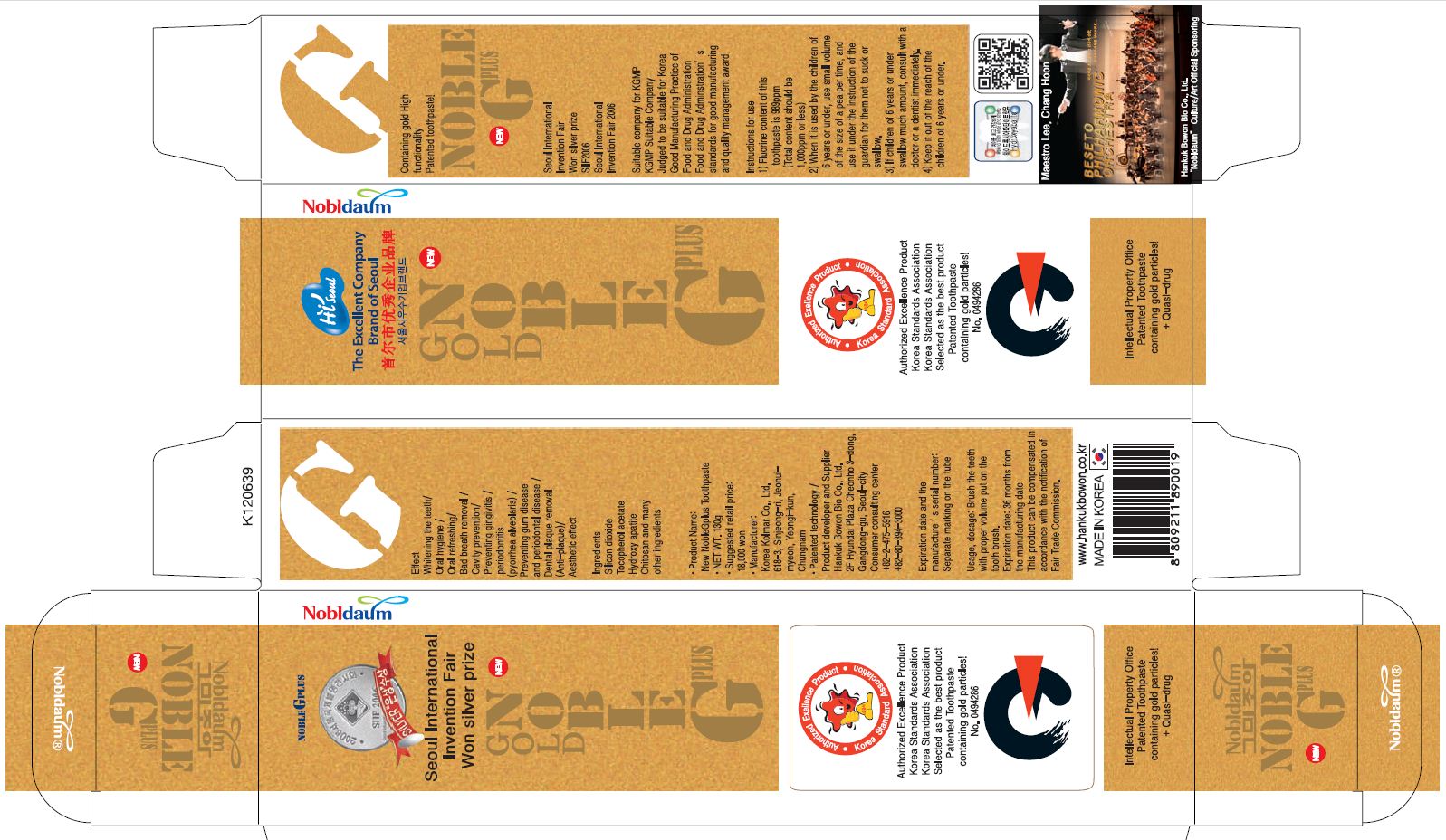
active ingredient: xylitol
silicone dioxide, sodium monofluorophosphate, hydrated silicone dioxide, d-sorbitol, carboxymethylcellulose sodium, sodium lauryl sulfate, sodium saccharin, gold leaf, green tea extract, polyethylene glycol 1500, L-menthol, hydroxyapatite, chitosan, stearic acid, polysorbate 80, water
whiten and strong teeth
removal of bad breath
prevention of gingivitis and periodontitis
prevention of periodontal diseases and gum diseases
removal of dental plaque
keep out of reach of the children
apply Proper Amount of the toothpaste on the tooth.
■ For tooth only.
■ Avoid contact with eyes.
■ Do not swallow. If swallowed, get medical help.
brush your teeth by putting appropriate amount of tooth paste