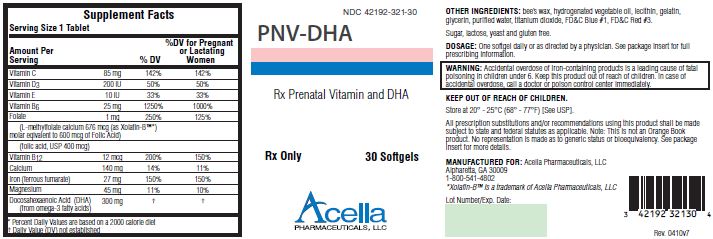
DESCRIPTION: PNV-DHA is a prescription prenatal/postnatal multivitamin/mineral/essential fatty acid softgel. Each softgel is blue in color, opaque and imprinted with “BP 321” on one side.
| Supplement Facts | |||
|---|---|---|---|
| Serving Size 1 Tablet | |||
| Amount Per
Serving | % DV | %DV for Pregnant
or Lactating Women |
|
| Vitamin C | 85 mg | 142% | 142% |
| Vitamin D 3 | 200 IU | 50% | 50% |
| Vitamin E | 10 IU | 33% | 33% |
| Vitamin D 6 | 25 mg | 1250% | 1000% |
| Folate | 1 mg | 250% | 125% |
| (L-methylfolate calcium 676 mcg (as Xolafin-B™
*)
molar equivalent to 600 mcg of Folic Acid) |
|||
| (folic acid, USP 400 mcg) | |||
| Vitamin B 12 | 12 mcg | 200% | 150% |
| Calcium | 140 mg | 14% | 11% |
| Iron (ferrous fumarate) | 27 mg | 150% | 150% |
| Magnesium | 45 mg | 11% | 10% |
| Docosahexaenoic Acid (DHA)
(from omega-3 fatty acids) | 300 mg | † | † |
OTHER INGREDIENTS: bee’s wax, hydrogenated vegetable oil, lecithin, gelatin, glycerin, purified water, titanium dioxide, FD&C Blue #1, FD&C Red #3.
WARNING: Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and International Normalized Ratio (INR). Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
PRECAUTIONS: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
ADVERSE REACTIONS: Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
DOSAGE AND ADMINISTRATION: Before, during and/or after pregnancy, one softgel daily or as directed by a physician.
HOW SUPPLIED: PNV-DHA is supplied in child-resistant bottles of 30 softgels (NDC# 42192-321-30).
Store at 20° - 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F) [See USP Controlled Room Temperature.]
All prescription substitutions and/or recommendations using this product shall be made subject to state and federal statutes as applicable. Please note: this is not an Orange Book product and has not been subjected to FDA therapeutic equivalency or other equivalency testing. No representation is made as to generic status or bioequivalency. Each person recommending a prescription substitution using this product shall make such recommendations based on each such person’s professional opinion and knowledge, upon evaluating the active ingredients, excipients, inactive ingredients and chemical information provided herein.