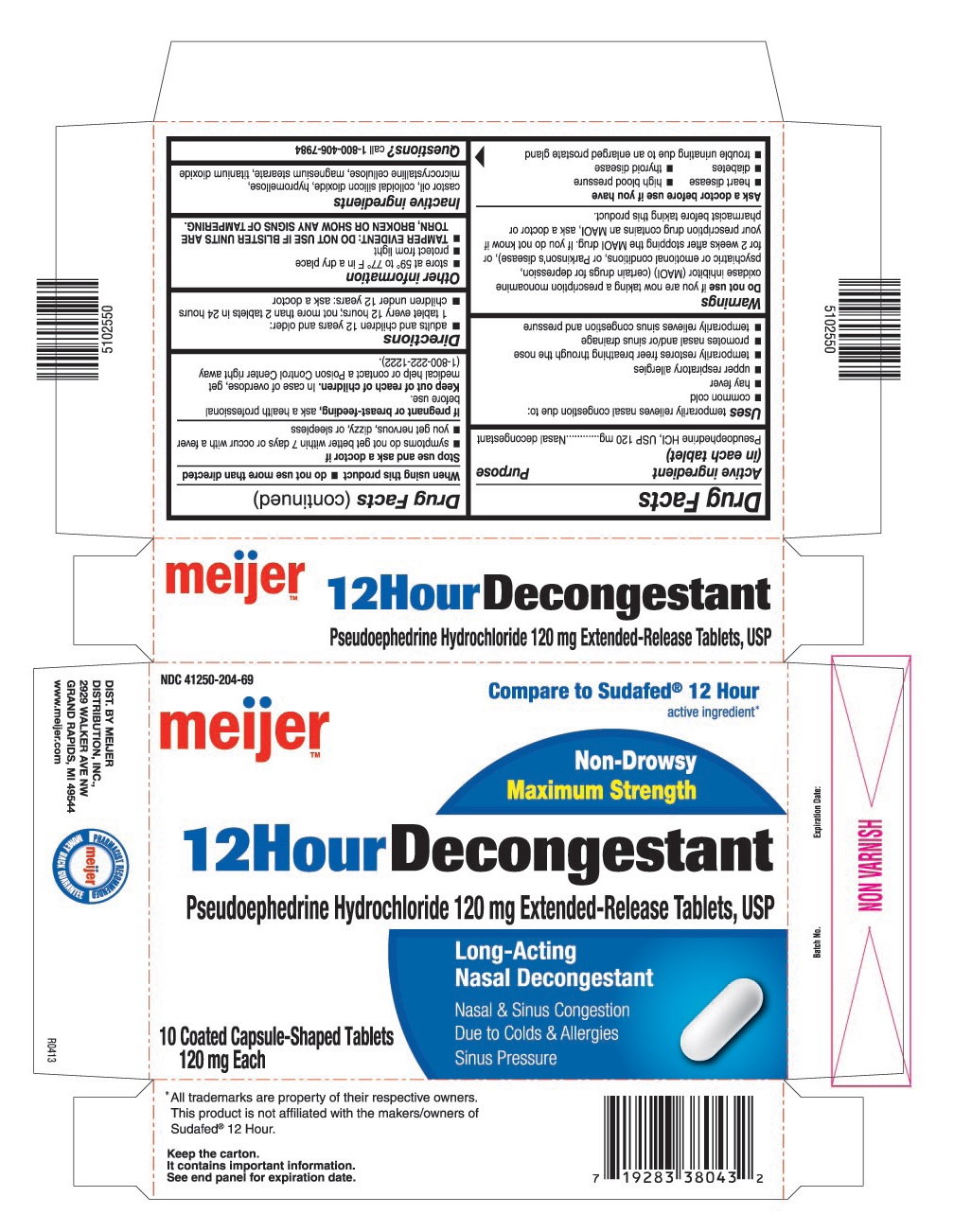
USES
Temporarily relieves nasal congestion due to:
- common cold
- hay fever
- upper respiratory allergies
- temporarily restores freer breathing through the nose
- promotes nasal and/or sinus drainage
- temporarily relieves sinus congestion and pressure
WARNINGS
Do not use
If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- diabetes
- thyroid disease
- trouble urinating due to an enlarged prostate gland
DIRECTIONS
- adults and children 12 years and older: 1 tablet every 12 hours; not more than 2 tablets in 24 hours
- children under 12 years: ask a doctor
OTHER INFORMATION
- store at 59° to 77° F in a dry place
- protect from light
- TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
INACTIVE INGREDIENTS
Castor oil, colloidal silicon dioxide, hypromellose, microcrystalline cellulose, magnesium stearate, titanium dioxide