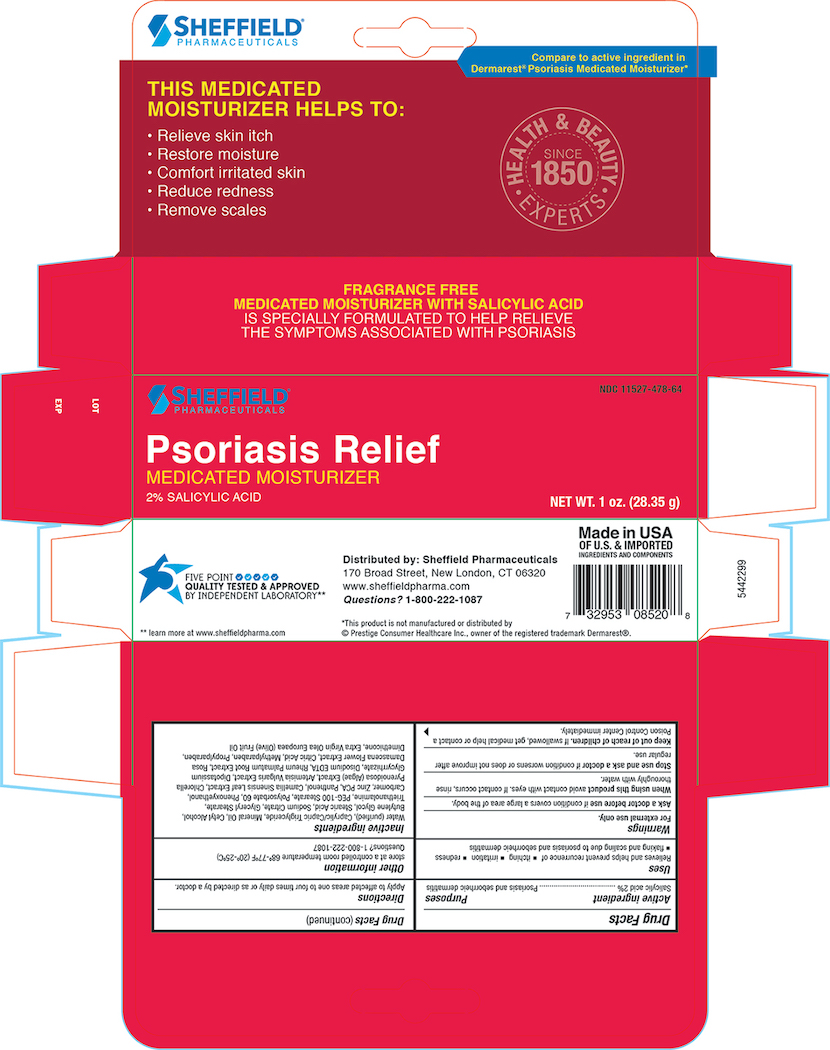
Uses
Relieves and helps prevent recurrence of
- itching
- irration
- redness
- flaking and scaling due to psoriasis and seborrheic dermatitis
Warnings
For external use only
Ask A doctor before use if condition covers a large area of the body.
When using this product avoid contact with eyes. if contact occurs, rinse throughly with water.
Stop use and ask a doctor if condition worsens or does not improve after regular use.
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Other information
store at a controlled room temperature 68°-77°F ( 20°- 25°C)
Questions? 1-800-222-1087
Inactive ingredients
Purifed Water, Caprylic/Capric Triglyceride, Mineral Oil, Cetyl Alcohol, Butylene Glycol, Stearic Acid, Sodium Citrate, Glyceral Stearate, Triethanolamine, PEG-100 Sterate, Polysorbate 60 , Phenoxyethanol, Carbomer, Zinc PCA, Panthenol, Camellia Oleifera leaf extract , Algea Extract, Artemisia Vulgaris Extract, Dipotassium Glycyrrhizate, Disodium EDTA, Rheum Palmatum Extract, Rosa Damascena Extract, Citric Acid, Methylparaben, Propylparaben, Dimethicone, Extra Virign Olea Europaea( Olive) Fruit oil