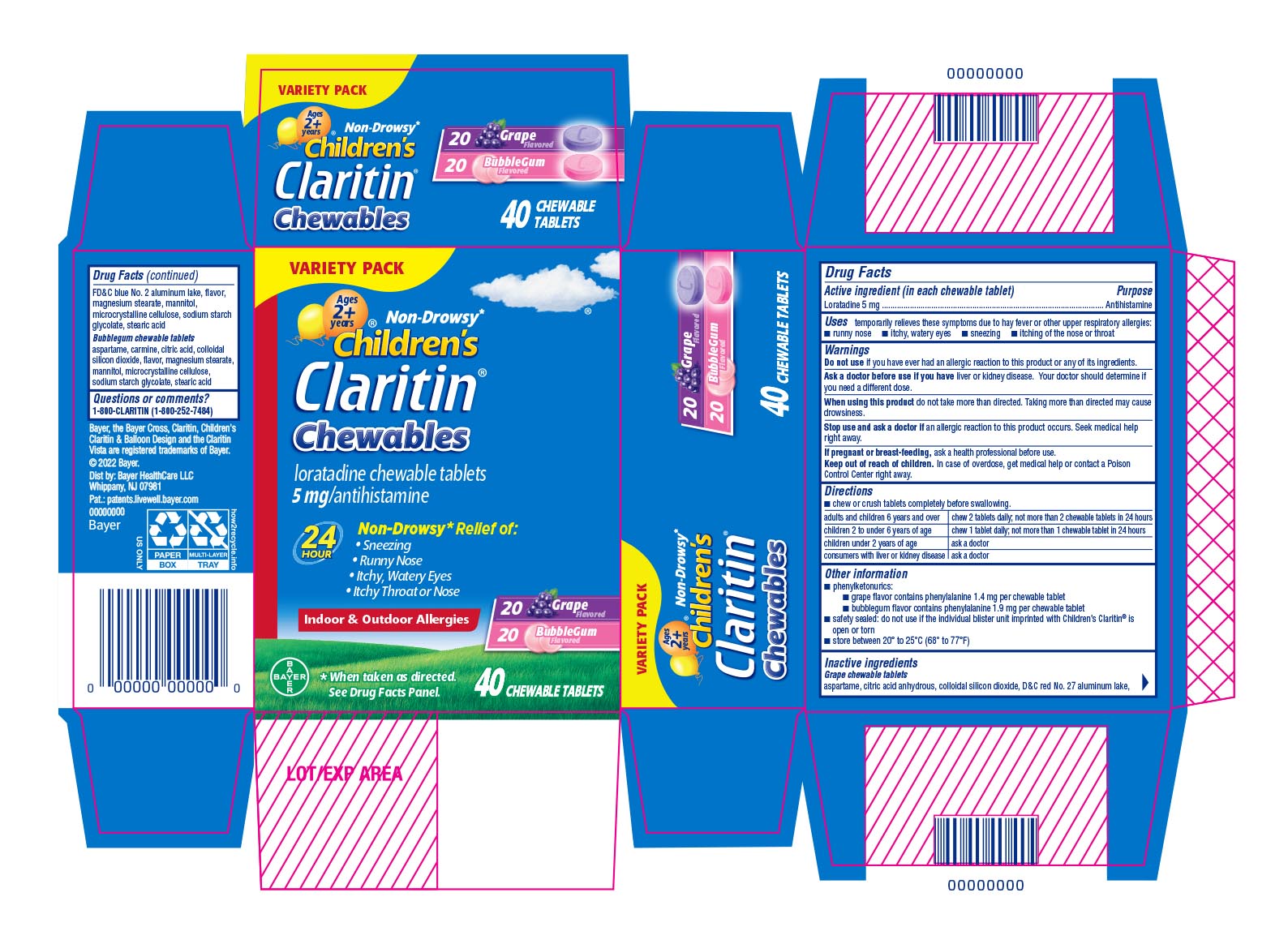
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
●runny nose ●itchy, watery eyes ●sneezing ●itching of the nose or throat
Warnings
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
Directions
- chew or crush tablets completely before swallowing.
| adults and children 6 years and over | chew 2 tablets daily; not more than 2 chewable tablets in 24 hours |
| children 2 to under 6 years of age | chew 1 tablet daily; not more than 1 chewable tablet in 24 hours |
| children under 2 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
Other Information
phenylketonurics:
- grape flavor contains phenylalanine 1.4 mg per chewable tablet
- bubblegum flavor contains phenylalanine 1.9 mg per chewable tablet
safety sealed: do not use if the individual blister unit imprinted with Children's Claritin® is open or torn
store between 20° to 25°C (68° to 77°F)
Inactive ingredients
Grape chewable tablets
aspartame, citric acid anhydrous, colloidal silicon dioxide, D&C red No. 27 aluminum lake, FD&C blue No. 2 aluminum lake, flavor, magnesium stearate, mannitol, microcrystalline cellulose, sodium starch glycolate, stearic aicd
Bubblegum chewable tablets
aspartame, carmine, citric acid, colloidal silicon dioxide, flavor, magnesium stearate, mannitol, microcrystalline cellulose, sodium starch glycolate, stearic acid
PRINCIPAL DISPLAY PANEL - 40 Chewable Tablet Blister Pack Carton
VARIETY PACK
ages
2 +
years
Non-Drowsy*
Children's
Claritin ®
Chewables
loratadine chewable tablets
5 mg/antihistamine
24 Hour
Non-Drowsy* Relief of:
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose
Indoor & Outdoor Allergies
20 Grape Flavored
20 Bubblegum Flavored
*When taken as directed. See Drug Facts Panel.
40 CHEWABLE TABLETS