FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
SHINGRIX is a vaccine indicated for prevention of herpes zoster (HZ) (shingles):
• in adults aged 50 years and older.
• in adults aged 18 years and older who are or will be at increased risk of HZ due to immunodeficiency or immunosuppression caused by known disease or therapy.
Limitations of Use:
- •
- SHINGRIX is not indicated for prevention of primary varicella infection (chickenpox).
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only.
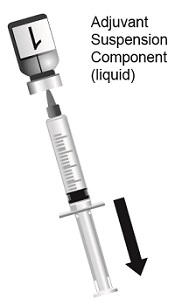
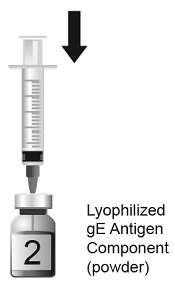
2.1 Reconstitution
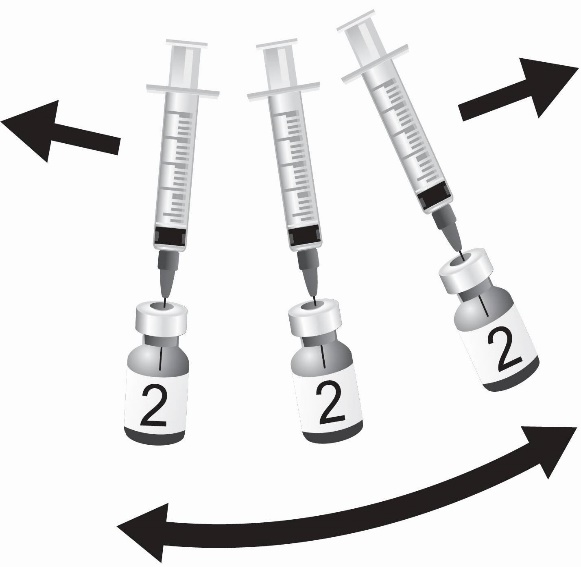
SHINGRIX is supplied in 2 vials that must be combined prior to administration. Prepare SHINGRIX by reconstituting the lyophilized varicella zoster virus glycoprotein E (gE) antigen component (powder) with the accompanying AS01B adjuvant suspension component (liquid). Use only the supplied adjuvant suspension component (liquid) for reconstitution. The reconstituted vaccine should be an opalescent, colorless to pale brownish liquid. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, the vaccine should not be administered.
2.2 Administration Instructions
For intramuscular injection only.
After reconstitution, administer SHINGRIX immediately or store refrigerated between 2° and 8°C (36° and 46°F) and use within 6 hours. Discard reconstituted vaccine if not used within 6 hours.
Use a separate sterile needle and sterile syringe for each individual. The preferred site for intramuscular injection is the deltoid region of the upper arm.
2.3 Dose and Schedule
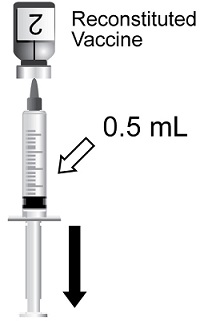
Two doses (0.5 mL each) administered intramuscularly according to the following schedules:
• A first dose at Month 0 followed by a second dose administered 2 to 6 months later.
• For individuals who are or will be immunodeficient or immunosuppressed and who would benefit from a shorter vaccination schedule: A first dose at Month 0 followed by a second dose administered 1 to 2 months later.
3 DOSAGE FORMS AND STRENGTHS
SHINGRIX is a suspension for injection supplied as a single-dose vial of lyophilized gE antigen component to be reconstituted with the accompanying vial of AS01B adjuvant suspension component. A single dose after reconstitution is 0.5 mL.
4 CONTRAINDICATIONS
Do not administer SHINGRIX to anyone with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine or after a previous dose of SHINGRIX [see Description (11)].
5 WARNINGS AND PRECAUTIONS
5.1 Preventing and Managing Allergic Vaccine Reactions
Prior to administration, the healthcare provider should review the immunization history for possible vaccine sensitivity and previous vaccination-related adverse reactions. Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of SHINGRIX.
5.2 Guillain-Barré Syndrome (GBS)
In a postmarketing observational study, an increased risk of GBS was observed during the 42 days following vaccination with SHINGRIX [see Adverse Reactions (6.2)].
5.3 Syncope
Syncope (fainting) can be associated with the administration of injectable vaccines, including SHINGRIX. Syncope can be accompanied by transient neurological signs such as visual disturbance, paresthesia, and tonic-clonic limb movements. Procedures should be in place to avoid falling injury and to restore cerebral perfusion following syncope.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared with rates in the clinical trials of another vaccine and may not reflect the rates observed in practice. There is the possibility that broad use of SHINGRIX could reveal adverse reactions not observed in clinical trials.
Adults Aged 50 Years and Older
Overall, 17,041 adults aged 50 years and older received at least 1 dose of SHINGRIX in 17 clinical studies.
The safety of SHINGRIX was evaluated by pooling data from 2 placebo-controlled clinical studies (Studies 1 and 2) involving 29,305 subjects aged 50 years and older who received at least 1 dose of SHINGRIX (n = 14,645) or saline placebo (n = 14,660) administered according to a 0- and 2-month schedule. At the time of vaccination, the mean age of the population was 69 years; 7,286 (25%) subjects were aged 50 to 59 years, 4,488 (15%) subjects were aged 60 to 69 years, and 17,531 (60%) subjects were aged 70 years and older. Both studies were conducted in North America, Latin America, Europe, Asia, and Australia. In the overall population, the majority of subjects were White (74%), followed by Asian (18%), Black (1.4%), and other racial/ethnic groups (6%); 58% were female.
Solicited Adverse Reactions: In Studies 1 and 2, data on solicited local and general adverse reactions were collected using standardized diary cards for 7 days following each vaccine dose or placebo (i.e., day of vaccination and the next 6 days) in a subset of subjects (n = 4,886 receiving SHINGRIX, n = 4,881 receiving placebo with at least 1 documented dose). Across both studies, the percentages of subjects aged 50 years and older reporting each solicited local and general adverse reaction following administration of SHINGRIX (both doses combined) were pain (78%), redness (38%), and swelling (26%); and myalgia (45%), fatigue (45%), headache (38%), shivering (27%), fever (21%), and gastrointestinal symptoms (17%).
The reported frequencies of specific solicited local adverse reactions and general adverse reactions (overall per subject), by age group, from the 2 studies are presented in Table 1.
| Total vaccinated cohort for safety included all subjects with at least 1 documented dose (n). a 7 days included day of vaccination and the subsequent 6 days. b Data for subjects aged 50 to 59 years and 60 to 69 years are based on Study 1. Data for subjects aged 70 years and older are based on pooled data from Study 1: NCT01165177 and Study 2: NCT01165229. c Placebo was a saline solution. d Grade 3 pain: Defined as significant pain at rest; prevents normal everyday activities. e Grade 3 myalgia, fatigue, headache, shivering, and GI: Defined as preventing normal activity. f Fever defined as ≥37.5°C/99.5°F for oral, axillary, or tympanic route, or ≥38°C/100.4°F for rectal route; Grade 3 fever defined as >39.0°C/102.2°F. g GI = Gastrointestinal symptoms including nausea, vomiting, diarrhea, and/or abdominal pain. |
||||||
|
Adverse Reactions |
Aged 50-59 Years |
Aged 60-69 Years |
Aged ≥70 Years |
|||
|
SHINGRIX |
Placeboc |
SHINGRIX |
Placeboc |
SHINGRIX |
Placeboc |
|
|
Local Adverse Reactions |
n = 1,315 % |
n = 1,312 % |
n = 1,311 % |
n = 1,305 % |
n = 2,258 % |
n = 2,263 % |
|
Pain |
88 |
14 |
83 |
11 |
69 |
9 |
|
Pain, Grade 3d |
10 |
1 |
7 |
1 |
4 |
0.2 |
|
Redness |
39 |
1 |
38 |
2 |
38 |
1 |
|
Redness, >100 mm |
3 |
0 |
3 |
0 |
3 |
0 |
|
Swelling |
31 |
1 |
27 |
1 |
23 |
1 |
|
Swelling, >100 mm |
1 |
0 |
1 |
0 |
1 |
0 |
|
General Adverse Reactions |
n = 1,315 % |
n = 1,312 % |
n = 1,309 % |
n = 1,305 % |
n = 2,252 % |
n = 2,264 % |
|
Myalgia |
57 |
15 |
49 |
11 |
35 |
10 |
|
Myalgia, Grade 3e |
9 |
1 |
5 |
1 |
3 |
0.4 |
|
Fatigue |
57 |
20 |
46 |
17 |
37 |
14 |
|
Fatigue, Grade 3e |
9 |
2 |
5 |
1 |
4 |
1 |
|
Headache |
51 |
22 |
40 |
16 |
29 |
12 |
|
Headache, Grade 3e |
6 |
2 |
4 |
0.2 |
2 |
0.4 |
|
Shivering |
36 |
7 |
30 |
6 |
20 |
5 |
|
Shivering, Grade 3e |
7 |
0.2 |
5 |
0.3 |
2 |
0.3 |
|
Fever |
28 |
3 |
24 |
3 |
14 |
3 |
|
Fever, Grade 3f |
0.4 |
0.2 |
1 |
0.2 |
0.1 |
0.1 |
|
GIg |
24 |
11 |
17 |
9 |
14 |
8 |
|
GI, Grade 3e |
2 |
1 |
1 |
1 |
1 |
0.4 |
The incidence of solicited local and general reactions was lower in subjects aged 70 years and older compared with those aged 50 to 69 years.
The local and general adverse reactions seen with SHINGRIX had a median duration of 2 to 3 days.
There were no differences in the proportions of subjects reporting any or Grade 3 solicited local reactions between Dose 1 and Dose 2. Headache and shivering were reported more frequently by subjects after Dose 2 (28% and 21%, respectively) compared with Dose 1 (24% and 14%, respectively). Grade 3 solicited general adverse reactions (headache, shivering, myalgia, and fatigue) were reported more frequently by subjects after Dose 2 (2.3%, 3%, 4%, and 4%, respectively) compared with Dose 1 (1.4%, 1.4%, 2.3%, and 2.4%, respectively).
Unsolicited Adverse Events: Unsolicited adverse events that occurred within 30 days following each vaccination (Day 0 to 29) were recorded on a diary card by all subjects. In the 2 studies, unsolicited adverse events occurring within 30 days of vaccination were reported in 51% and 32% of subjects who received SHINGRIX (n = 14,645) or placebo (n = 14,660), respectively (Total Vaccinated Cohort). Unsolicited adverse events that occurred in ≥1% of recipients of SHINGRIX and at a rate at least 1.5-fold higher than placebo included chills (4% versus 0.2%), injection site pruritus (2.2% versus 0.2%), malaise (1.7% versus 0.3%), arthralgia (1.7% versus 1.2%), nausea (1.4% versus 0.5%), and dizziness (1.2% versus 0.8%).
Gout (including gouty arthritis) was reported by 0.18% (n = 27) versus 0.05% (n = 8) of subjects who received SHINGRIX or placebo, respectively, within 30 days of vaccination; available information is insufficient to determine a causal relationship with SHINGRIX.
Serious Adverse Events (SAEs): In the 2 studies, SAEs were reported at similar rates in subjects who received SHINGRIX (2.3%) or placebo (2.2%) from the first administered dose up to 30 days post-last vaccination. SAEs were reported for 10.1% of subjects who received SHINGRIX and for 10.4% of subjects who received placebo from the first administered dose up to 1 year post-last vaccination. One subject (<0.01%) reported lymphadenitis and 1 subject (<0.01%) reported fever greater than 39°C; there was a basis for a causal relationship with SHINGRIX.
Optic ischemic neuropathy was reported in 3 subjects (0.02%) who received SHINGRIX (all within 50 days after vaccination) and 0 subjects who received placebo; available information is insufficient to determine a causal relationship with SHINGRIX.
Deaths: From the first administered dose up to 30 days post-last vaccination, deaths were reported for 0.04% of subjects who received SHINGRIX and 0.05% of subjects who received placebo in the 2 studies. From the first administered dose up to 1 year post-last vaccination, deaths were reported for 0.8% of subjects who received SHINGRIX and for 0.9% of subjects who received placebo. Causes of death among subjects were consistent with those generally reported in adult and elderly populations.
Potential Immune-Mediated Diseases: In the 2 studies, new onset potential immune-mediated diseases (pIMDs) or exacerbation of existing pIMDs were reported for 0.6% of subjects who received SHINGRIX and 0.7% of subjects who received placebo from the first administered dose up to 1 year post-last vaccination. The most frequently reported pIMDs occurred with comparable frequencies in the group receiving SHINGRIX and the placebo group.
Dosing Schedule: In an open-label clinical study, 238 subjects aged 50 years and older received SHINGRIX as a 0- and 2-month or 0- and 6-month schedule. The safety profile of SHINGRIX was similar when administered according to a 0- and 2-month or 0- and 6-month schedule and was consistent with that observed in Studies 1 and 2.
Immunocompromised Adults Aged 18 Years and Older
The safety of SHINGRIX was evaluated in 6 placebo-controlled clinical studies that enrolled 3,116 subjects aged 18 years and older from 5 different immunodeficient or immunosuppressed (referred to as immunocompromised) populations, in which a total of 1,587 received SHINGRIX. In all studies, subjects received Doses 1 and 2 of SHINGRIX 1 to 2 months apart. Safety monitoring for these studies was similar to Studies 1 and 2. In addition, subjects were monitored for events relevant to their specific disease or condition.
At the time of receipt of SHINGRIX or placebo, the mean age of the population was 55 years; 28% subjects were aged 18 to 49 years and 72% subjects were aged 50 years and older. Each of the studies was conducted in one or more of the following regions: North America, Latin America, Europe, Asia, Africa, and Australia/New Zealand. The majority of subjects were White (77%), followed by Asian (17%), Black (2%), and other racial groups (3%); 4% were of American Hispanic or Latino ethnicity; 37% were female.
| a The first dose was administered within 50 to 70 days after autologous hematopoietic stem cell transplantation. b Safety follow-up was driven by HZ case accrual and ranged from a minimum of 12 months post last vaccination to 4 years at subject level. c For subjects who were vaccinated during a cancer therapy course, each dose was administered with at least 10 days between vaccination and cancer therapy cycles. d For subjects who received the vaccination after a full cancer therapy course, the first dose was administered from 10 days to 6 months after cancer therapy had ended. e The first dose was administered between 4 to 18 months after renal transplantation. f In the PreChemo group (TVC: SHINGRIX [n = 90], placebo [n = 91]), the first dose was administered a maximum of 1 month to a minimum of 10 days before the start of a chemotherapy cycle, and the second dose was administered on the first day of a chemotherapy cycle. g In the OnChemo group (TVC: SHINGRIX [n = 27], placebo [n = 24]), each dose was administered on the first day of a chemotherapy cycle. |
|||||||||||
|
Clinical Studies |
Number of Subjects Vaccinated |
Study Population |
Safety Follow-up Period |
||||||||
|
SHINGRIX |
Placebo |
||||||||||
|
auHSCT (NCT01610414) |
922 |
924 |
Autologous hematopoietic stem cell transplant recipientsa |
29 months median safety follow-upb |
|||||||
|
Hematologic Malignancies (NCT01767467) |
283 |
279 |
Hematologic malignanciesc,d |
12 months post last vaccination |
|||||||
|
Renal Transplant (NCT02058589) |
132 |
132 |
Renal transplant recipientse |
12 months post last vaccination |
|||||||
|
Solid Malignant |
117 |
115 |
Solid tumors receiving chemotherapyf,g |
12 months post last vaccination |
|||||||
|
HIV (NCT01165203) |
74 |
49 |
HIV-infected subjects |
12 months post last vaccination |
|||||||
|
auHSCT (NCT00920218) |
59 |
30 |
Autologous hematopoietic stem cell transplant recipientsa |
12 months post last vaccination |
|||||||
In the auHSCT study (NCT01610414), at the time of receipt of SHINGRIX or placebo, the mean age of the population was 55 years; 25% of subjects were aged 18 to 49 years and 75% subjects were aged 50 years and older. The majority of subjects were White (78%), followed by Asian (16%), Black (2%), and other racial groups (3%); 3% were of American Hispanic or Latino ethnicity; 37% were female.
Solicited Adverse Reactions: Solicited local adverse reactions reported within 7 days following administration of SHINGRIX (both doses combined) in auHSCT recipients (aged 18 to 49 and ≥50 years of age) were pain (88% and 83%), redness (30% and 35%), and swelling (21% and 18%). Solicited general adverse reactions reported within 7 days following administration of SHINGRIX (both doses combined) in auHSCT recipients (aged 18 to 49 and ≥50 years of age) were fatigue (64% and 54%), myalgia (58% and 52%), headache (44% and 30%), gastrointestinal symptoms (21% and 28%), shivering (31% and 25%), and fever (28% and 18%). The percentages of subjects aged 18 years and older reporting each solicited local and general adverse reaction following administration of each dose of SHINGRIX or placebo in the auHSCT study (NCT01610414) are presented in Table 3.
| Total vaccinated cohort (TVC) for safety included all subjects with at least 1 documented dose (n). % = Percentage of subjects reporting the symptom at least once. a 7 days included day of vaccination and the subsequent 6 days. b Placebo was sucrose reconstituted with saline solution. c Grade 3 pain: defined as significant pain at rest preventing normal everyday activities. d Grade 3 myalgia, fatigue, headache, shivering, and GI: defined as preventing normal activity. e GI = Gastrointestinal symptoms including nausea, vomiting, diarrhea, and/or abdominal pain. |
||||||||||||||||||||
|
Adverse Reactions |
Aged 18-49 Years |
Aged ≥50 Years |
||||||||||||||||||
|
SHINGRIX |
Placebob |
SHINGRIX |
Placebob |
|||||||||||||||||
|
Dose 1 |
Dose 2 |
Dose 1 |
Dose 2 |
Dose 1 |
Dose 2 |
Dose 1 |
Dose 2 |
|||||||||||||
|
Local Adverse Reactions |
n = 223
|
n = 205
|
n = 217
|
n = 207
|
n = 673
|
n = 635
|
n = 673
|
n = 627
|
||||||||||||
|
Pain |
81 |
82 |
8 |
6 |
75 |
74 |
6 |
5 |
||||||||||||
|
Pain, Grade 3c |
11 |
11 |
1 |
0 |
5 |
7 |
0.3 |
0 |
||||||||||||
|
Redness |
20 |
25 |
0 |
0 |
21 |
28 |
1 |
1 |
||||||||||||
|
Redness, >100 mm |
1 |
2 |
0 |
0 |
1 |
3 |
0 |
0 |
||||||||||||
|
Swelling |
14 |
17 |
0 |
0 |
10 |
15 |
1 |
1 |
||||||||||||
|
Swelling, >100 mm |
0 |
2 |
0 |
0 |
0.1 |
1 |
0 |
0 |
||||||||||||
|
General Adverse Reactions |
n = 222
|
n = 203 % |
n = 218
|
n = 207
|
n = 674
|
n = 633
|
n = 674
|
n = 628
|
||||||||||||
|
Myalgia |
41 |
51 |
22 |
21 |
37 |
43 |
18 |
17 |
||||||||||||
|
Myalgia, Grade 3d |
4 |
8 |
2 |
2 |
2 |
4 |
1 |
1 |
||||||||||||
|
Fatigue |
49 |
51 |
34 |
25 |
37 |
46 |
31 |
26 |
||||||||||||
|
Fatigue, Grade 3d |
6 |
10 |
1 |
2 |
3 |
4 |
2 |
3 |
||||||||||||
|
Headache |
23 |
38 |
17 |
17 |
15 |
25 |
13 |
8 |
||||||||||||
|
Headache, Grade 3d |
1 |
5 |
0 |
2 |
0.1 |
2 |
0.4 |
1 |
||||||||||||
|
Shivering |
20 |
26 |
12 |
6 |
11 |
21 |
7 |
7 |
||||||||||||
|
Shivering, Grade 3d |
1 |
6 |
0 |
0 |
0.4 |
3 |
1 |
0.2 |
||||||||||||
|
Fever, ≥37.5°C/99.5°F |
9 |
28 |
4 |
2 |
6 |
15 |
3 |
4 |
||||||||||||
|
Fever, Grade 3 >39.5°C/103.1°F |
0 |
1 |
0 |
0 |
0.1 |
0.2 |
0 |
0.2 |
||||||||||||
|
GIe |
14 |
13 |
13 |
12 |
18 |
18 |
16 |
12 |
||||||||||||
|
GI, Grade 3d |
1 |
1 |
0 |
1 |
1 |
2 |
1 |
2 |
||||||||||||
In general, the reported frequencies of solicited local and general adverse reactions in the other studies in immunocompromised populations were similar to that in the auHSCT study (NCT01610414). The local and general adverse reactions seen with SHINGRIX had a median duration of 1 to 3 days across all studies enrolling immunocompromised subjects.
Unsolicited Adverse Events: Across all 6 studies enrolling immunocompromised subjects, unsolicited adverse events, including both serious and non-serious events, occurring within 30 days following each vaccination were reported in 46% and 44% of subjects who received SHINGRIX or placebo. Adverse events of arthralgia, infective pneumonia, and influenza-like illness occurred in ≥1% of recipients of SHINGRIX and at a rate at least 1.5-fold higher than placebo (1.5% versus 1.0%, 1.5% versus 0.9%, and 1.3% versus 0.6%, respectively).
Serious Adverse Events: Across all 6 studies enrolling immunocompromised subjects, SAEs were reported at similar rates in subjects who received SHINGRIX (7%) or placebo (8%) from the first administered dose up to 30 days post-last vaccination. SAEs were reported for 26% of subjects who received SHINGRIX and for 27% of subjects who received placebo from the first administered dose up to 1 year post-last vaccination. SAEs of infective pneumonia were reported for 21 subjects (1.3%) who received SHINGRIX and for 11 subjects (0.7%) who received placebo up to 30 days post-last vaccination. Available information is insufficient to determine a causal relationship to vaccination.
Deaths: Across all 6 studies enrolling immunocompromised subjects, from the first administered dose up to 30 days post-last vaccination, deaths were reported for 2 subjects (0.1%) who received SHINGRIX and 7 subjects (0.5%) who received placebo. From the first administered dose up to 1 year post-last vaccination, deaths were reported for 6% of subjects who received SHINGRIX and for 6% of subjects who received placebo. Causes of death among subjects were consistent with those expected in the populations evaluated.
Potential Immune-Mediated Diseases: Across all 6 studies enrolling immunocompromised subjects, new onset pIMDs or exacerbation of existing pIMDs were reported for 1.3% of subjects who received SHINGRIX and 1.0% of subjects who received placebo from the first administered dose up to 1 year post-last vaccination. There were no notable imbalances in specific pIMDs between treatment groups.
Other Medically Relevant Events: In the auHSCT study (NCT01610414), relapse or progression was reported by 315 of 922 subjects (34%) who received at least one dose of SHINGRIX and 331 of 924 subjects (36%) who received placebo from the first vaccination to study end.
In the auHSCT study (NCT00920218), relapse or progression was reported by 17 of 59 subjects (29%) who received at least one dose of SHINGRIX and 8 of 30 subjects (27%) who received placebo from the first vaccination to study end.
In the hematologic malignancy study, relapse or progression was reported by 45 of 283 subjects (16%) who received at least one dose of SHINGRIX and 58 of 279 subjects (21%) who received placebo from the first vaccination to study end.
In the renal transplant study, biopsy-confirmed allograft rejection was reported by 4 of 132 (3%) of subjects who received SHINGRIX and by 7 of 132 (5%) of subjects who received placebo from the first vaccination to study end (approximately 13 months later). Creatinine as a measure of graft function and changes in alloimmunity post-vaccination were not systematically evaluated.
In the HIV study, at least 1 event of worsening HIV condition was reported by 9 of 74 (12%) of subjects who received SHINGRIX and by 5 of 49 (10%) of subjects who received placebo from the first vaccination to study end.
Concomitant Administration with 23-Valent Pneumococcal Polysaccharide Vaccine
In an open-label clinical study (NCT02045836) in subjects aged 50 years and older, information about solicited local and systemic adverse reactions was collected using diary cards for 7 days (i.e., day of vaccination and the next 6 days). When PNEUMOVAX 23 was co-administered with the first dose of SHINGRIX compared to when the first dose of SHINGRIX was given alone, a greater percentage of subjects reported fever, defined as ≥37.5°C/99.5°F (16% vs. 7%, respectively) and shivering (21% vs. 7%, respectively) [see Clinical Studies (14.7)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of SHINGRIX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the vaccine.
General Disorders and Administration Site Conditions
Decreased mobility of the injected arm which may persist for 1 or more weeks.
Immune System Disorders
Hypersensitivity reactions, including angioedema, rash, and urticaria.
Nervous System Disorders
Guillain-Barré syndrome.
Postmarketing Observational Study of the Risk of Guillain-Barré Syndrome following Vaccination with SHINGRIX
The association between vaccination with SHINGRIX and GBS was evaluated among Medicare beneficiaries aged 65 years or older. Using Medicare claims data, from October 2017 through February 2020, vaccinations with SHINGRIX among beneficiaries were identified through National Drug Codes, and potential cases of hospitalized GBS among recipients of SHINGRIX were identified through International Classification of Diseases codes.
The risk of GBS following vaccination with SHINGRIX was assessed in self-controlled case series analyses using a risk window of 1 to 42 days post-vaccination and a control window of 43 to 183 days post-vaccination. The primary analysis (claims-based, all doses) found an increased risk of GBS during the 42 days following vaccination with SHINGRIX, with an estimated 3 excess cases of GBS per million doses administered to adults aged 65 years or older. In secondary analyses, an increased risk of GBS was observed during the 42 days following the first dose of SHINGRIX, with an estimated 6 excess cases of GBS per million doses administered to adults aged 65 years or older, and no increased risk of GBS was observed following the second dose of SHINGRIX. These analyses of GBS diagnoses in claims data were supported by analyses of GBS cases confirmed by medical record review. While the results of this observational study suggest a causal association of GBS with SHINGRIX, available evidence is insufficient to establish a causal relationship.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The data are insufficient to establish if there is vaccine-associated risk with SHINGRIX in pregnant women.
A developmental toxicity study was performed in female rats administered SHINGRIX or the AS01B adjuvant alone prior to mating, during gestation, and during lactation. The total dose was 0.2 mL on each occasion (a single human dose of SHINGRIX is 0.5 mL). This study revealed no adverse effects on fetal or pre-weaning development due to SHINGRIX (see Data).
Data
Animal Data: In a developmental toxicity study, female rats were administered SHINGRIX or the AS01B adjuvant alone by intramuscular injection 28 and 14 days prior to mating, on gestation Days 3, 8, 11, and 15, and on lactation Day 7. The total dose was 0.2 mL on each occasion (a single human dose of SHINGRIX is 0.5 mL). No adverse effects on pre-weaning development up to post-natal Day 25 were observed. There were no vaccine-related fetal malformations or variations.
8.2 Lactation
Risk Summary
It is not known whether SHINGRIX is excreted in human milk. Data are not available to assess the effects of SHINGRIX on the breastfed infant or on milk production/excretion.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SHINGRIX and any potential adverse effects on the breastfed child from SHINGRIX or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
Safety and effectiveness in individuals younger than 18 years have not been established. SHINGRIX is not indicated for prevention of primary varicella infection (chickenpox).
8.5 Geriatric Use
Adults Aged 60 Years and Older
Of the total number of subjects who received at least 1 dose of SHINGRIX in Studies 1 and 2 (n = 14,645), 2,243 (15%) were aged 60 to 69 years, 6,837 (47%) were aged 70 to 79 years, and 1,921 (13%) were aged 80 years and older. There were no clinically meaningful differences in efficacy across the age groups. [See Clinical Studies (14.1, 14.2, 14.3).]
The frequencies of solicited local and general adverse reactions in subjects aged 70 years and older were lower than in younger adults (aged 50 through 69 years). [See Adverse Reactions (6.1).]
Immunocompromised Adults Aged 65 Years and Older
Of the total number of subjects who received at least 1 dose of SHINGRIX in the auHSCT study (n = 922), 172 (18.7%) were aged 65 years and older [see Clinical Studies (14.5)]. There were no clinically meaningful differences in efficacy between these subjects and younger adults (aged 18 through 64 years).
Of the total number of subjects who received at least 1 dose of SHINGRIX across the 6 studies in immunocompromised subjects (n = 1,587), 337 (21.2%) were aged 65 years and older. The frequencies of solicited local and general adverse reactions in subjects aged 65 years and older were generally similar to or lower than those reported by younger adults (aged 18 through 64 years).
11 DESCRIPTION
SHINGRIX (Zoster Vaccine Recombinant, Adjuvanted) is a sterile suspension for intramuscular injection. The vaccine is supplied as a vial of lyophilized recombinant varicella zoster virus surface glycoprotein E (gE) antigen component, which must be reconstituted at the time of use with the accompanying vial of AS01B adjuvant suspension component. The lyophilized gE antigen component is presented in the form of a sterile white powder. The AS01B adjuvant suspension component is an opalescent, colorless to pale brownish liquid supplied in vials.
The gE antigen is obtained by culturing genetically engineered Chinese Hamster Ovary cells, which carry a truncated gE gene, in media containing amino acids, with no albumin, antibiotics, or animal-derived proteins. The gE protein is purified by several chromatographic steps, formulated with excipients, filled into vials, and lyophilized.
The adjuvant suspension component is AS01B which is composed of 3-O-desacyl-4’-monophosphoryl lipid A (MPL) from Salmonella minnesota and QS-21, a saponin purified from plant extract Quillaja saponaria Molina, combined in a liposomal formulation. The liposomes are composed of dioleoyl phosphatidylcholine (DOPC) and cholesterol in phosphate-buffered saline solution containing disodium phosphate anhydrous, potassium dihydrogen phosphate, sodium chloride, and water for injection.
After reconstitution, each 0.5‑mL dose is formulated to contain 50 mcg of the recombinant gE antigen, 50 mcg of MPL, and 50 mcg of QS-21. Each dose also contains 20 mg of sucrose (as stabilizer), 4.385 mg of sodium chloride, 1 mg of DOPC, 0.54 mg of potassium dihydrogen phosphate, 0.25 mg of cholesterol, 0.160 mg of sodium dihydrogen phosphate dihydrate, 0.15 mg of disodium phosphate anhydrous, 0.116 mg of dipotassium phosphate, and 0.08 mg of polysorbate 80. After reconstitution, SHINGRIX is a sterile, opalescent, colorless to pale brownish liquid.
SHINGRIX does not contain preservatives. Each dose may also contain residual amounts of host cell proteins (≤3.0%) and DNA (≤2.1 picograms) from the manufacturing process.
The vial stoppers are not made with natural rubber latex.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The risk of developing HZ, which increases with age and with immunosuppression due to disease and/or therapy, appears to be related to a decline in varicella zoster virus (VZV)-specific immunity. SHINGRIX was shown to boost VZV-specific immune response, which is thought to be the mechanism by which it protects against zoster disease [see Clinical Studies (14)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
SHINGRIX has not been evaluated for its carcinogenic or mutagenic potential. Vaccination of female rats with SHINGRIX had no effect on fertility [see Use in Specific Populations (8.1)]. In a male fertility study, rats were vaccinated with 0.1 mL of SHINGRIX (a single human dose is 0.5 mL) on 42, 28, and 14 days prior to mating. There were no effects on male fertility.
14 CLINICAL STUDIES
14.1 Efficacy in Subjects Aged 50 Years and Older
Study 1 was a randomized, placebo-controlled, observer-blind clinical study conducted in 18 countries. Randomization was stratified (8:5:3:1) by age: 50 to 59 years, 60 to 69 years, 70 to 79 years, and ≥80 years. The study excluded, among others, subjects who were immunocompromised, had a history of previous HZ, were vaccinated against varicella or HZ, and patients whose survival was not expected to be at least 4 years or with conditions that might interfere with study evaluations. Subjects were followed for the development of HZ and postherpetic neuralgia (PHN) for a median of 3.1 years (range: 0 to 3.7 years). Suspected HZ cases were followed prospectively for the development of PHN, an HZ-related complication defined as HZ-associated pain (rated as 3 or greater on a 0- to 10-point scale by the study subject) occurring or persisting at least 90 days following the onset of rash in confirmed cases of HZ.
The primary efficacy analysis population (referred to as the modified Total Vaccinated Cohort [mTVC]) included 14,759 subjects aged 50 years and older who received 2 doses (0 and 2 months) of either SHINGRIX (n = 7,344) or placebo (n = 7,415) and did not develop a confirmed case of HZ within 1 month after the second dose. In the mTVC population, 61% were female; 72% were White, 19% were Asian, 1.7% were Black, and 7% were of other racial/ethnic groups. The mean age of subjects was 62.3 years.
Confirmed HZ cases were determined by either Polymerase Chain Reaction (PCR) (89.4%) or by a Clinical Evaluation Committee (10.6%).
Efficacy against Herpes Zoster
Compared with placebo, SHINGRIX significantly reduced the risk of developing HZ by 97.2% (95% CI: 93.7, 99.0) in subjects aged 50 years and older (Table 4).
| N = Number of subjects included in each group; n = Number of subjects having at least 1 confirmed HZ episode; HZ = Herpes zoster; CI = Confidence Interval. a Study 1: NCT01165177. b mTVC = Modified Total Vaccinated Cohort defined as subjects who received 2 doses (0 and 2 months) of either SHINGRIX or placebo and did not develop a confirmed case of HZ within 1 month after the second dose. c Primary study endpoint was based on confirmed HZ cases in subjects aged 50 years and older. |
|||||||
|
Age Group (Years) |
SHINGRIX |
Placebo |
% Efficacy (95% CI) |
||||
|
N |
n |
Incidence Rate of HZ per 1,000 Person-Years |
N |
n |
Incidence Rate of HZ per 1,000 Person-Years | ||
|
Overall (≥50)c |
7,344 |
6 |
0.3 |
7,415 |
210 |
9.1 |
97.2 (93.7, 99.0) |
|
50-59 |
3,492 |
3 |
0.3 |
3,525 |
87 |
7.8 |
96.6 (89.6, 99.3) |
|
60-69 |
2,141 |
2 |
0.3 |
2,166 |
75 |
10.8 |
97.4 (90.1, 99.7) |
|
≥70 |
1,711 |
1 |
0.2 |
1,724 |
48 |
9.4 |
97.9 (87.9, 100.0) |
In a descriptive analysis, vaccine efficacy against HZ in subjects aged 50 years and older was 93.1% (95% CI: 81.3, 98.2) in the fourth year post-vaccination.
Occurrence of Postherpetic Neuralgia
Among all subjects aged 50 years or older in the mTVC, no cases of PHN were reported in the vaccine group compared with 18 cases reported in the placebo group.
14.2 Efficacy in Subjects Aged 70 Years and Older
Study 2 was a randomized, placebo-controlled, observer-blind clinical study conducted in 18 countries. Randomization was stratified (3:1) by age: 70 to 79 years and ≥80 years. With the exception of age, the study exclusion criteria were the same as for Study 1. Subjects were followed for the development of HZ and PHN for a median of 3.9 years (range: 0 to 4.5 years). Suspected HZ cases were followed prospectively for the development of PHN as for Study 1.
The primary efficacy analysis population (mTVC) included 13,163 subjects aged 70 years and older who received 2 doses (0 and 2 months) of either SHINGRIX (n = 6,541) or placebo (n = 6,622) and did not develop a confirmed case of HZ within 1 month after the second dose. In the mTVC population, 55% were female; 78% were White, 17% were Asian, 1% were Black, and 4% were of other racial/ethnic groups. The mean age of subjects was 75.5 years.
Confirmed HZ cases were determined by either PCR (92.3%) or by a Clinical Evaluation Committee (7.7%).
Efficacy against Herpes Zoster
Vaccine efficacy results against HZ in subjects aged 70 years and older are shown in Table 5.
| N = Number of subjects included in each group; n = Number of subjects having at least 1 confirmed HZ episode; HZ = Herpes zoster; CI = Confidence Interval. a Study 2: NCT01165229. b mTVC = Modified Total Vaccinated Cohort defined as subjects who received 2 doses (0 and 2 months) of either SHINGRIX or placebo and did not develop a confirmed case of HZ within 1 month after the second dose. c Primary study endpoint was based on confirmed HZ cases in subjects aged 70 years and older. |
|||||||
|
Age Group (Years) |
SHINGRIX |
Placebo |
% Efficacy (95% CI) |
||||
|
N |
n |
Incidence Rate of HZ per 1,000 Person-Years |
N |
n |
Incidence Rate of HZ per 1,000 Person-Years | ||
|
Overall (≥70)c |
6,541 |
23 |
0.9 |
6,622 |
223 |
9.2 |
89.8 (84.3, 93.7) |
|
70-79 |
5,114 |
17 |
0.9 |
5,189 |
169 |
8.8 |
90.0 (83.5, 94.3) |
|
≥80 |
1,427 |
6 |
1.2 |
1,433 |
54 |
11.0 |
89.1 (74.7, 96.2) |
In a descriptive analysis, vaccine efficacy against HZ in subjects aged 70 years and older was 85.1% (95% CI: 64.5, 94.8) in the fourth year after vaccination.
Efficacy against Postherpetic Neuralgia
Among all subjects aged 70 years or older in the mTVC, 4 cases of PHN were reported in the vaccine group compared with 28 cases reported in the placebo group. Vaccine efficacy against PHN was 85.5% (95% CI: [58.5; 96.3]). The benefit of SHINGRIX in the prevention of PHN can be attributed to the effect of the vaccine on the prevention of HZ.
Reduction of Use of Pain Medication
Among subjects with confirmed HZ, the use of HZ-associated pain medications was reported for 10 of 23 subjects (43.5%) who received SHINGRIX and for 160 of 223 subjects (71.7%) who received placebo.
14.3 Pooled Efficacy Analyses across Studies 1 and 2
The efficacy of SHINGRIX to prevent HZ and PHN in subjects aged 70 years and older was evaluated by combining the results from Studies 1 and 2 through a pre-specified pooled analysis in the mTVC. A total of 8,250 and 8,346 subjects who received SHINGRIX and placebo, respectively, were included in the pooled mTVC analysis.
Efficacy against Herpes Zoster
Compared with placebo, SHINGRIX significantly reduced the risk of developing HZ by 91.3% (95% CI: 86.9, 94.5) in subjects aged 70 years and older (Table 6).
| N = Number of subjects included in each group; n = Number of subjects having at least 1 confirmed HZ episode; HZ = Herpes zoster; CI = Confidence Interval. a Pooled data from Study 1: NCT01165177 (subjects ≥50 years) and Study 2: NCT01165229 (subjects ≥70 years). b mTVC = Modified Total Vaccinated Cohort defined as subjects who received 2 doses (0 and 2 months) of either SHINGRIX or placebo and did not develop a confirmed case of HZ within 1 month after the second dose. c Primary endpoint of pooled analysis was based on confirmed HZ cases in subjects aged 70 years and older. |
|||||||
|
Age Group (Years) |
SHINGRIX |
Placebo |
% Efficacy (95% CI) |
||||
|
N |
n |
Incidence Rate of HZ per 1,000 Person-Years |
N |
n |
Incidence Rate of HZ per 1,000 Person-Years | ||
|
Overall (≥70)c |
8,250 |
25 |
0.8 |
8,346 |
284 |
9.3 |
91.3 (86.9, 94.5) |
|
70-79 |
6,468 |
19 |
0.8 |
6,554 |
216 |
8.9 |
91.3 (86.0, 94.9) |
|
≥80 |
1,782 |
6 |
1.0 |
1,792 |
68 |
11.1 |
91.4 (80.2, 96.9) |
Efficacy against Postherpetic Neuralgia
Table 7 compares the overall rates of PHN in the vaccine and placebo groups across both studies.
| N = Number of subjects included in each group; n = Number of subjects having at least 1 PHN; CI = Confidence Interval. a Pooled data from Study 1: NCT01165177 (subjects ≥50 years) and Study 2: NCT01165229 (subjects ≥70 years). b mTVC = Modified Total Vaccinated Cohort defined as subjects who received 2 doses (0 and 2 months) of either SHINGRIX or placebo and did not develop a confirmed case of HZ within 1 month after the second dose. c PHN = Postherpetic neuralgia defined as HZ-associated pain rated as 3 or greater (on a 0- to 10-point scale) occurring or persisting at least 90 days following the onset of rash using Zoster Brief Pain Inventory questionnaire. |
|||||||||||||||
|
Age Group (Years) |
SHINGRIX |
Placebo |
% Efficacy (95% CI) |
||||||||||||
|
N |
n |
Incidence Rate of PHNc per 1,000 Person-Years |
N |
n |
Incidence Rate of PHN per 1,000 Person-Years | ||||||||||
|
Overall (≥70) |
8,250 |
4 |
0.1 |
8,346 |
36 |
1.2 |
88.8 (68.7, 97.1) |
||||||||
|
70-79 |
6,468 |
2 |
0.1 |
6,554 |
29 |
1.2 |
93.0 (72.5, 99.2) |
||||||||
|
≥80 |
1,782 |
2 |
0.3 |
1,792 |
7 |
1.1 |
71.2 (-51.5, 97.1) |
||||||||
The benefit of SHINGRIX in the prevention of PHN can be attributed to the effect of the vaccine on the prevention of HZ. The efficacy of SHINGRIX in the prevention of PHN in subjects with confirmed HZ could not be demonstrated.
14.4 Immunological Evaluation to Support Dosing Schedule
A measure of the immune response that confers protection against HZ is unknown. Anti-gE antibody levels were measured by anti-gE enzyme-linked immunosorbent assay (gE ELISA) and were used to support the dosing schedule.
In an open-label clinical study, 238 subjects aged 50 years and older received SHINGRIX on either a 0- and 2-month or 0- and 6-month schedule. Non-inferiority of the 0- and 6-month schedule compared with the 0- and 2-month schedule based on anti-gE ELISA GMCs 1 month after the second dose was demonstrated.
14.5 Efficacy in Immunocompromised Adults Aged 18 Years and Older
The efficacy of SHINGRIX was evaluated in one Phase 3 randomized, placebo-controlled, observer-blind clinical study in immunocompromised adults aged ≥18 years who received an auHSCT 50 to 70 days prior to Dose 1 and who were expected to receive prophylactic antiviral therapy for ≤6 months post-transplant. The efficacy of SHINGRIX was calculated post-hoc in another randomized, placebo-controlled, observer-blind study in subjects with hematologic malignancies who received Dose 1 of SHINGRIX or placebo during or within 6 months of completing immunosuppressive chemotherapy. Each of these studies was conducted in the following regions: North America, Latin America, Europe, Asia, Africa (auHSCT study only), and Australia/New Zealand.
Efficacy in Subjects Aged 18 Years and Older: auHSCT Recipients
In the auHSCT study, subjects were followed for the development of HZ and PHN for a median of 21 months (range: 0 to 49.4 months). Suspected HZ cases were followed prospectively for the development of PHN as in Studies 1 and 2.
The primary efficacy analysis population (mTVC) for the auHSCT study included 1,721 subjects who received 2 doses of either SHINGRIX or placebo and did not develop a confirmed case of HZ within 1 month after the second dose. Confirmed HZ cases were determined by either PCR (83.7%) or by a Clinical Evaluation Committee (16.3%).
Efficacy against Herpes Zoster: Compared with placebo, SHINGRIX significantly reduced the risk of developing HZ in auHSCT recipients aged 18 years and older (Table 8).
| auHSCT = Autologous, hematopoietic, stem cell transplant. N = Number of subjects included in each group; n = Number of subjects having at least 1 confirmed HZ episode; HZ = Herpes zoster; CI = Confidence Interval. a mTVC = Modified Total Vaccinated Cohort, defined as subjects who received 2 doses (0 and 1 to 2 months) of either SHINGRIX or placebo and did not develop a confirmed case of HZ within 1 month after the second dose. Follow-up was censored at the time of treatment for relapse. b NCT01610414. c Primary study endpoint was based on confirmed HZ cases in subjects aged ≥18 years. |
||||||||
|
Clinical Studies |
Age Group (Years) |
SHINGRIX |
Placebo |
% Efficacy (95% CI) |
||||
|
N |
n |
Incidence Rate of HZ per 1,000 Person-Years |
N |
n |
Incidence Rate of HZ per 1,000 Person-Years | |||
|
auHSCTb |
Overall (≥18)c |
870 |
49 |
30.0 |
851 |
135 |
94.3 |
68.2 (55.5, 77.6) |
|
18-49 |
213 |
9 |
21.5 |
212 |
29 |
76.0 |
71.8 (38.7, 88.3) |
|
|
≥50 |
657 |
40 |
33.0 |
639 |
106 |
100.9 |
67.3 (52.6, 77.9) |
|
Efficacy in Subjects Aged 18 Years and Older with Hematologic Malignancies
In the study of hematologic malignancies, the mean age was 57 years. The majority of subjects were White (71%), followed by Asian (25%), Black (0.4%), and other racial groups (4%); 5% were of American Hispanic or Latino ethnicity; and 41% were female. Subjects were followed for the development of HZ for a median of 11.1 months (range: 0 to 15.6 months). PHN was not assessed as a study endpoint.
In the hematologic malignancy study, the population for the post hoc efficacy analysis included 515 subjects who received 2 doses of either SHINGRIX or placebo and did not develop a confirmed case of HZ within 1 month after the second dose. Confirmed HZ cases were determined by either PCR (81.3%) or by a Clinical Evaluation Committee (18.7%). The post hoc analysis showed SHINGRIX was 87.2% (95% CI [44.2; 98.6]) effective against development of HZ. The incidence rate of HZ per 1,000 person-years was 8.5 versus 66.2 in the SHINGRIX and placebo groups, respectively.
Additional Efficacy Endpoints Evaluated in the auHSCT Study
Efficacy against Postherpetic Neuralgia: In a descriptive analysis, including all subjects aged ≥18 years in the mTVC, 1 case of PHN was reported in the vaccine group compared with 9 cases reported in the placebo group. Vaccine efficacy against PHN was 89.3% (95% CI: [22.5; 99.8]). The benefit of SHINGRIX in the prevention of PHN can be attributed to the effect of the vaccine on the prevention of HZ.
Herpes Zoster-Associated Pain: Subjects with suspected HZ rated their “worst” HZ-associated pain on a 10-point scale. Among subjects with confirmed HZ, 37 out of 49 subjects (75.5%) receiving SHINGRIX and 120 out of 135 subjects (88.9%) receiving placebo rated their “worst” HZ-associated pain as 3 or greater. In this subset of subjects, the median duration of “worst” HZ associated pain was 14 and 24 days, among SHINGRIX and placebo recipients, respectively.
14.6 Revaccination after Vaccination with ZOSTAVAX (Zoster Vaccine Live)
In an open-label clinical study (NCT02581410), subjects aged 65 years and older, who had been previously vaccinated with ZOSTAVAX more than 5 years prior to study enrollment (n = 215) or who had never been vaccinated with ZOSTAVAX (n = 215), received 1 dose of SHINGRIX at Months 0 and 2. Subjects who had never been vaccinated with ZOSTAVAX were matched to those who had been previously vaccinated with ZOSTAVAX according to the predefined variables of age (65 to 69, 70 to 79, and ≥80 years), sex, race/ethnicity, and medical condition (immune-mediated diseases, diabetes mellitus, depression, pulmonary conditions, or heart conditions). The mean age was 71 years; 51% were female. All subjects were White and were not Hispanic or Latino.
The anti-gE antibody (Ab) concentration measured by ELISA 1 month following 2 doses of SHINGRIX in subjects who had previously been vaccinated with ZOSTAVAX was non-inferior to that of subjects who had never been vaccinated with ZOSTAVAX. The upper limit (UL) of the 95% confidence interval (CI) was 1.17 (success criterion <1.5) for the anti-gE Ab adjusted geometric mean concentration (GMC) ratio between subjects who had never been vaccinated with ZOSTAVAX and subjects who had been previously vaccinated with ZOSTAVAX. There was no evidence for interference in the immune response to SHINGRIX in subjects previously vaccinated with ZOSTAVAX.
14.7 Concomitant Administration with Other Vaccines
Concomitant Administration with Influenza Vaccine
In an open-label clinical study (NCT01954251), subjects aged 50 years and older received 1 dose each of SHINGRIX and quadrivalent influenza vaccine (FLUARIX QUADRIVALENT) at Month 0 and 1 dose of SHINGRIX at Month 2 (n = 413), or 1 dose of FLUARIX QUADRIVALENT at Month 0 and 1 dose of SHINGRIX at Months 2 and 4 (n = 415). The mean age of the population was 63 years; 52% were female. The majority of subjects were White (92%), followed by Asian (6%), and Black (2%); 0.4% were of American Hispanic or Latino ethnicity. There was no evidence for interference in the immune response to any of the antigens contained in SHINGRIX or the coadministered vaccine.
Concomitant Administration with PNEUMOVAX 23 (Pneumococcal Vaccine Polyvalent)
In an open-label clinical study (NCT02045836), subjects aged 50 years and older received 1 dose each of SHINGRIX and PNEUMOVAX 23 at Month 0 and 1 dose of SHINGRIX at Month 2 (n = 432), or 1 dose of PNEUMOVAX 23 at Month 0 and 1 dose of SHINGRIX at Months 2 and 4 (n = 433). The mean age of the population was 63 years; 60% were female. The majority of subjects were White (94%), followed by Black (2%), Asian (2%), and other racial groups (2%); 1% were of American Hispanic or Latino ethnicity.
The immune response to SHINGRIX, based on anti-gE Ab, was measured by ELISA 1 month after administration of the second dose of SHINGRIX. Immune responses to 12 of the 23 pneumococcal serotypes contained in PNEUMOVAX 23 were measured by multiplex opsonophagocytosis assay (MOPA) at 1 month after administration of the single dose of PNEUMOVAX 23. There was no evidence for interference in the immune response to the antigen contained in SHINGRIX or to the 12 evaluated antigens contained in PNEUMOVAX 23 when the two vaccines were administered concomitantly.
Concomitant Administration with PREVNAR 13 (Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM197 Protein])
In an open-label clinical study (NCT03439657), subjects aged 50 years and older received 1 dose each of SHINGRIX and PREVNAR 13 at Month 0 and 1 dose of SHINGRIX at Month 2 (n = 449), or 1 dose of PREVNAR 13 at Month 0 and 1 dose of SHINGRIX at Months 2 and 4 (n = 463). The mean age of the population was 63 years; 60% were female. The majority of subjects were White (98%), followed by Black (2%); 0.4% were of American Hispanic or Latino ethnicity.
The immune response to SHINGRIX, based on anti-gE Ab, was measured by ELISA 1 month after administration of the second dose of SHINGRIX. Immune responses to the pneumococcal serotypes contained in PREVNAR 13 were measured by MOPA at 1 month after administration of the single dose of PREVNAR 13. There was no evidence for interference in the immune response to the antigens contained in SHINGRIX or PREVNAR 13 when the two vaccines were administered concomitantly.
Concomitant Administration with BOOSTRIX (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Adsorbed)
In an open-label clinical study (NCT02052596), subjects aged 50 years and older received 1 dose each of SHINGRIX and BOOSTRIX at Month 0 and 1 dose of SHINGRIX at Month 2 (n = 412; concomitant administration group), or 1 dose of BOOSTRIX at Month 0 and 1 dose of SHINGRIX at Months 2 and 4 (n = 418; sequential administration group). The mean age of the population was 63 years; 54% were female. The majority of subjects were White (87%), followed by Black (11%), and other racial groups; 2% were of American Hispanic or Latino ethnicity.
The immune response to SHINGRIX, based on anti-gE Ab, was measured by ELISA 1 month after administration of the second dose of SHINGRIX. The immune response to BOOSTRIX (anti-D, anti-T, and antibodies to pertussis antigens) was measured 1 month after administration of the single dose of BOOSTRIX. Concomitant administration showed no evidence for interference in the immune response to the antigen contained in SHINGRIX or the antigens contained in BOOSTRIX, with the exception of one of the pertussis antigens (pertactin), which did not meet the non-inferiority criterion: the UL of the 95% CI for the adjusted GMC ratio (sequential administration group/concomitant administration group) for anti-pertactin antibody was 1.58 (non-inferiority criterion <1.5). The clinical significance of the reduced immune response to pertactin is unknown.
16 HOW SUPPLIED/STORAGE AND HANDLING
SHINGRIX is supplied as 2 components: A single-dose vial of lyophilized gE antigen component (powder) and a single-dose vial of adjuvant suspension component (liquid) (packaged without syringes or needles).
|
Presentation |
Carton NDC Number |
Components |
|
|
Adjuvant Suspension Component (liquid) |
Lyophilized gE Antigen Component (powder) |
||
|
An outer carton of 1 dose |
58160-819-12 |
Vial 1 of 2 NDC 58160-829-01 |
Vial 2 of 2 NDC 58160-828-01 |
|
An outer carton of 10 doses |
58160-823-11 |
10 vials NDC 58160-829-03 |
10 vials NDC 58160-828-03 |
16.1 Storage before Reconstitution
Adjuvant suspension component vials: Store refrigerated between 2° and 8°C (36° and 46°F). Protect vials from light. Do not freeze. Discard if the adjuvant suspension has been frozen.
Lyophilized gE antigen component vials: Store refrigerated between 2° and 8°C (36° and 46°F). Protect vials from light. Do not freeze. Discard if the antigen component has been frozen.
17 PATIENT COUNSELING INFORMATION
- •
- Inform patients of the potential benefits and risks of immunization with SHINGRIX and of the importance of completing the 2-dose immunization series according to the schedule.
- •
- Inform patients about the potential for adverse reactions that have been temporally associated with administration of SHINGRIX.
- •
- Provide the Vaccine Information Statements, which are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
BOOSTRIX and FLUARIX QUADRIVALENT are trademarks owned by or licensed to the GSK group of companies. The other brands listed are trademarks owned by or licensed to their owners and are not owned by or licensed to the GSK group of companies. The makers of these brands are not affiliated with and do not endorse the GSK group of companies or its products.
Manufactured by GlaxoSmithKline Biologicals
Rixensart, Belgium, U.S. License 1617, and
Distributed by GlaxoSmithKline
Durham, NC 27701
©2023 GSK group of companies or its licensor.
SHX:7PI
PRINCIPAL DISPLAY PANEL
NDC 58160-819-12
SHINGRIX
Zoster Vaccine Recombinant, Adjuvanted
Rx only
NOTICE: One vial of lyophilized powder and one vial of liquid suspension MUST BE COMBINED BEFORE USE
For 50 Years of Age and Older and For 18 Years of Age and Older who are Immunocompromised and at Increased Risk of Herpes Zoster
Contents (a single dose of SHINGRIX):
1 Vial containing Lyophilized gE Antigen Component
1 Vial containing Adjuvant Suspension Component
After reconstitution, a single dose of SHINGRIX is 0.5 mL
GSK
SHINGRIX
Antigen and Adjuvant Made in Belgium
©2021 GSK group of companies or its licensor.
- Rev. 7/23
- 515720
PRINCIPAL DISPLAY PANEL
NDC 58160-823-11
SHINGRIX
Zoster Vaccine Recombinant, Adjuvanted
Rx only
NOTICE: One vial of lyophilized powder and one vial of liquid suspension MUST BE COMBINED BEFORE USE
For 50 Years of Age and Older and For 18 Years of Age and Older who are Immunocompromised and at Increased Risk of Herpes Zoster
Contents (10 doses of SHINGRIX):
10 Vials containing Lyophilized gE Antigen Component
10 Vials containing Adjuvant Suspension Component
After reconstitution, a single dose of SHINGRIX is 0.5 mL
GSK
SHINGRIX
Antigen and Adjuvant Made in Belgium
©2023 GSK group of companies or its licensor.
- Rev. 8/23
- 515300