CISATRACURIUM BESYLATE- cisatracurium besylate injection
Hikma Farmaceutica
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CISATRACURIUM BESYLATE INJECTION safely and effectively. See full prescribing information for CISATRACURIUM BESYLATE INJECTION.
CISATRACURIUM BESYLATE injection for intravenous use Initial U.S. Approval: 1995 Revised: 2/2024 |
FULL PRESCRIBING INFORMATION: CONTENTS*ADVERSE REACTIONS
|
FULL PRESCRIBING INFORMATION
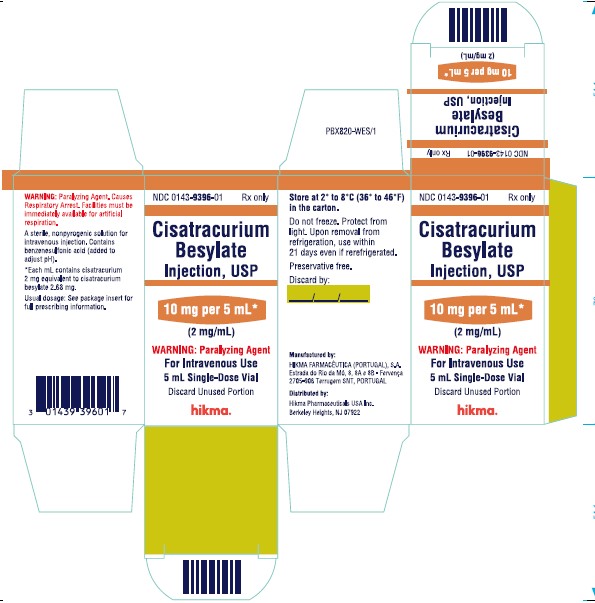
PRINCIPAL DISPLAY PANEL
NDC 0143-9396-01 Rx only
Cisatracurium Besylate Injection, USP
10 mg per 5 mL
(2 mg/mL)
WARNING: Paralyzing Agent
For Intravenous Use
5 mL Single-Dose Vial

NDC 0143-9396-01 Rx only
Cisatracurium Besylate Injection, USP
10 mg per 5 mL
(2 mg/mL)
WARNING: Paralyzing Agent
For Intravenous Use
5 mL Single-Dose Vial x 1
| CISATRACURIUM BESYLATE
cisatracurium besylate injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hikma Farmaceutica (452742943) |
| Registrant - Hikma Pharmaceuticals USA Inc. (001230762) |
Revised: 2/2024
Document Id: 2a5216c8-0277-430c-a4de-d058f38a7b46
Set id: 0273051b-fbeb-451c-b240-c524101181f4
Version: 7
Effective Time: 20240209
Hikma Farmaceutica