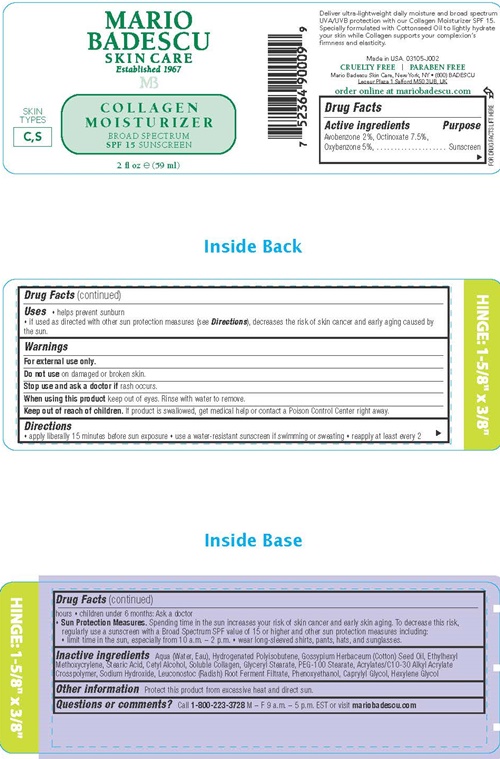
Uses
- helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early aging caused by the sun.
Warnings
For external use only.
Do not use on damaged or broken skin.
Stop use and ask a doctor if rash occurs.
When using this product keep out of eyes. Rinse with water to remove
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
- use a water-resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: Ask a doctor
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses.
Inactive ingredients
Aqua (Water, Eau), Hydrogenated Polysobutene, Gossyplum Herbaceum (Cotton) Seed Oil, Ethylhexyl Methoxycrylene, Stearic Acid, Cetyl Alcohol, Soluble Collagen, Glyceryl Stearate, PEG-100 stearate, Acrylates/C10-30 Acrylate Crosspolymer, Sodium Hydroxide, Leuconostoc (Radish) Root Ferment Filtrate, Phenoxyethanol, Caprylyl Glycol, Hexylene Glycol
Mario Badescu Collagen Moisturizer SPF 15 product label
MARIO
BADESCU
SKIN CARE
Established 1967
MB
COLLAGEN
MOISTURIZER
BROAD SPECTRUM
SPF 15 SUNSCREEN
SKIN
TYPES
C,S
2 fl oz (59 ml)
Deliver ultra-lightweight daily moisture and broad spectrum UVA/UVB protection with our Collagen Moisturizer SPF 15. Specially formulated with Cottonseed Oil to lightly hydrate your skin while Collagen supports your complexion's firmness and elasticity
Made in the USA. 03105-J002
CRUELTY FREE l PARABEN FREE
Mario Badescu Skin Care, New York, NY • (800) BADESCU
Leceur Plaza 1 Salford M50 3UB, UK
order online at mario badescu.com