Active ingredient
Brimonidine tartrate (0.025%)
Use
- •
- relieves redness of the eye due to minor eye irritations
Warnings
For external use only
Do not use
- •
- if solution changes color or becomes cloudy
Stop use and ask a doctor if
- •
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- •
- condition worsens or persists for more than 3 days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- •
- adults and children 5 years of age and over:
- •
- instill 1 drop in the affected eye(s) every 6-8 hours
- •
- do not use more than 4 times daily
- •
- remove contact lenses before use
- •
- wait at least 10 minutes before re-inserting contact lenses after use
- •
- if using other ophthalmic products while using this product, wait at least 5 minutes between each product
- •
- to avoid contamination, do not touch tip of container to any surface
- •
- replace cap after each use
- •
- children under 5 years of age: consult a doctor
Other information
- •
- store at 15-25°C (59-77°F)
Inactive ingredients
benzalkonium chloride, boric acid, calcium chloride dihydrate, glycerin, potassium chloride, sodium borate decahydrate, sodium chloride, water for injection. Hydrochloric acid and/or sodium hydroxide may be used to adjust pH.
Questions or comments?
Call: 1-800-553-5340
PRINCIPAL DISPLAY PANEL - 7.5 mL Carton
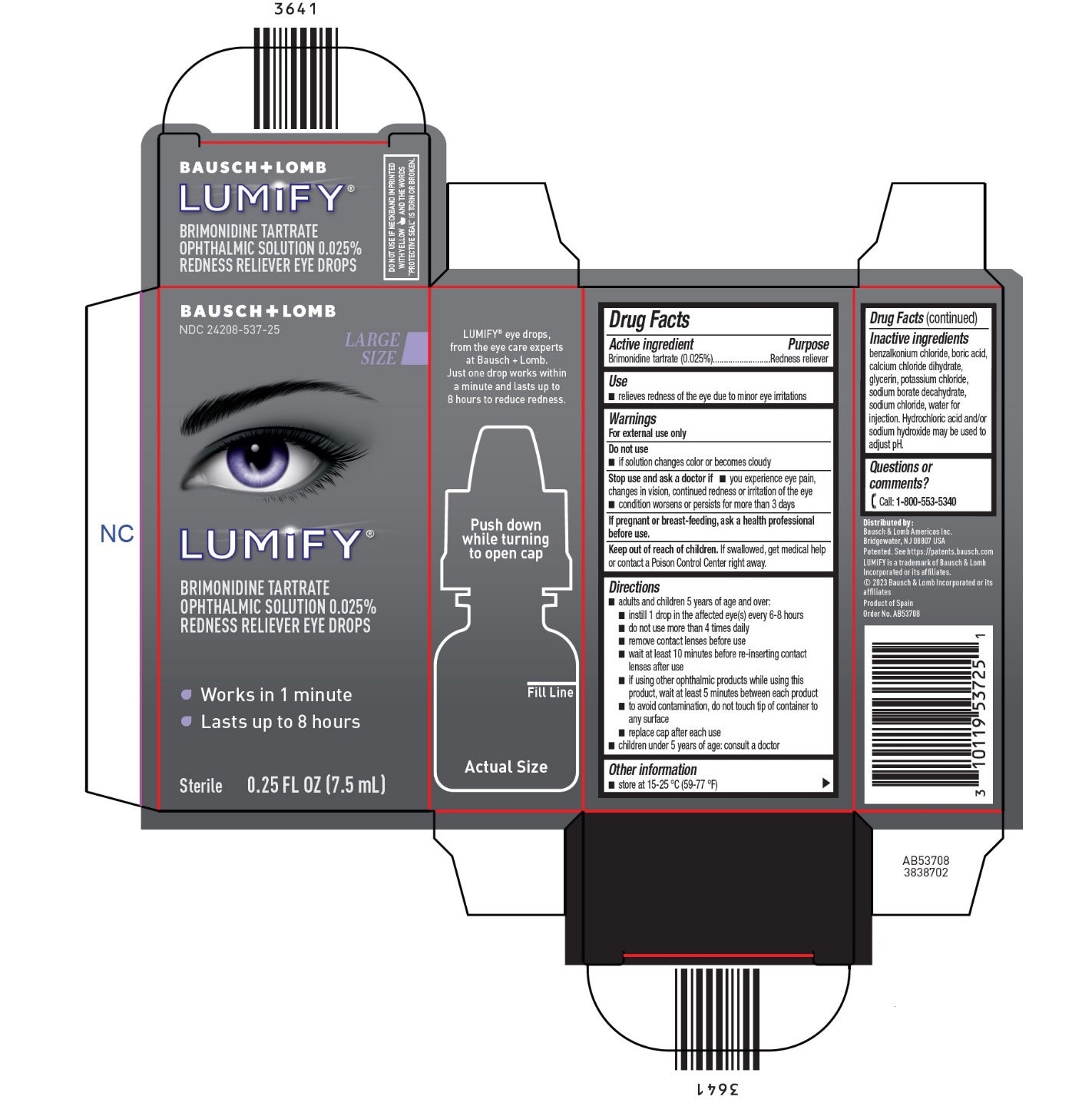 BAUSCH + LOMB
BAUSCH + LOMB
NDC 24208-537-25
LARGE
SIZE
LUMIFY®
BRIMONIDINE TARTRATE
OPHTHALMIC SOLUTION 0.025%
REDNESS RELIEVER EYE DROPS
- •
-
Works in 1 minute
- •
-
Lasts up to 8 hours
Sterile 0.25 FL OZ (7.5 mL)
AB53708
3838702
 BAUSCH + LOMB
BAUSCH + LOMB