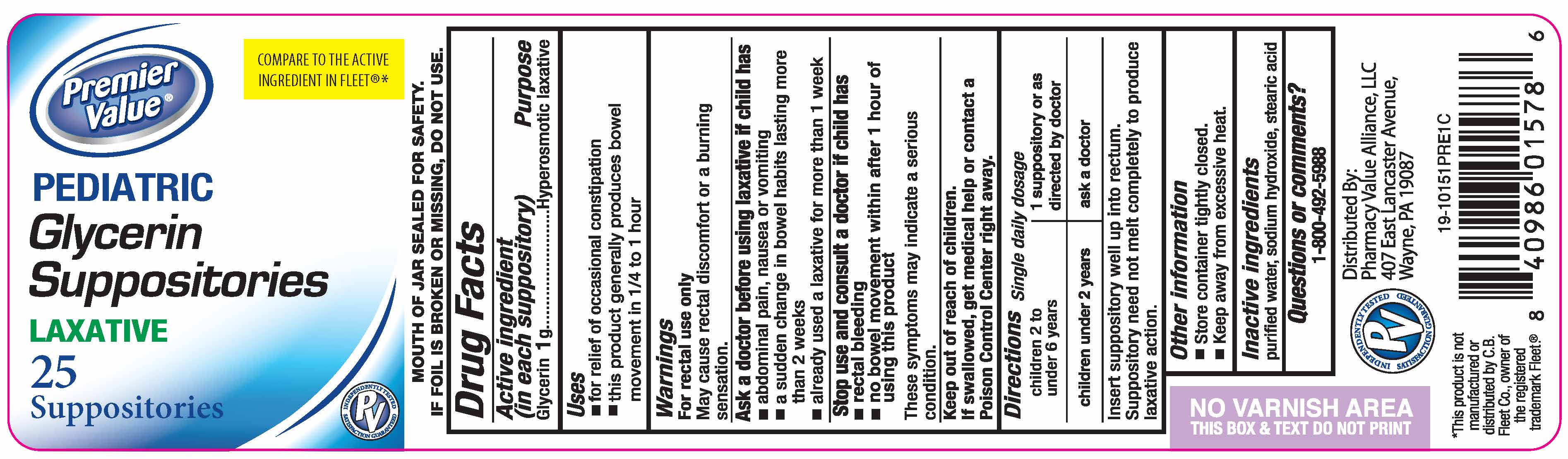
Uses
- for relief of occasional constipation
- this product generally produces a bowel movement in 1/4 to 1 hour
Warnings
For rectal use only
May cause rectal discomfort of burning sensation
Ask a Doctor before using laxative if child has
- abdominal pain, nausea or vomiting
- a sudden change in bowel habits lasting more than two weeks
- already used a laxative for more than 1 week
Directions
| children 2 to under 6 years | 1 suppository or as directed by a doctor |
| children under 2 years | ask a doctor |
Insert suppository well up intorectum.
Suppository need not melt completely to produce laxative action.
Pediatric Glycerin Suppositories, 25 count
PremVal722.jpg
The product package shown above represents a sample of that currently in use. Additional packaging may also be available.
PediatricGlycerin Suppositories, 25 count
Distributed by Pharmacy Value Alliance, LLC
407 East Lancaster Avenue
Wayne, PA 19087