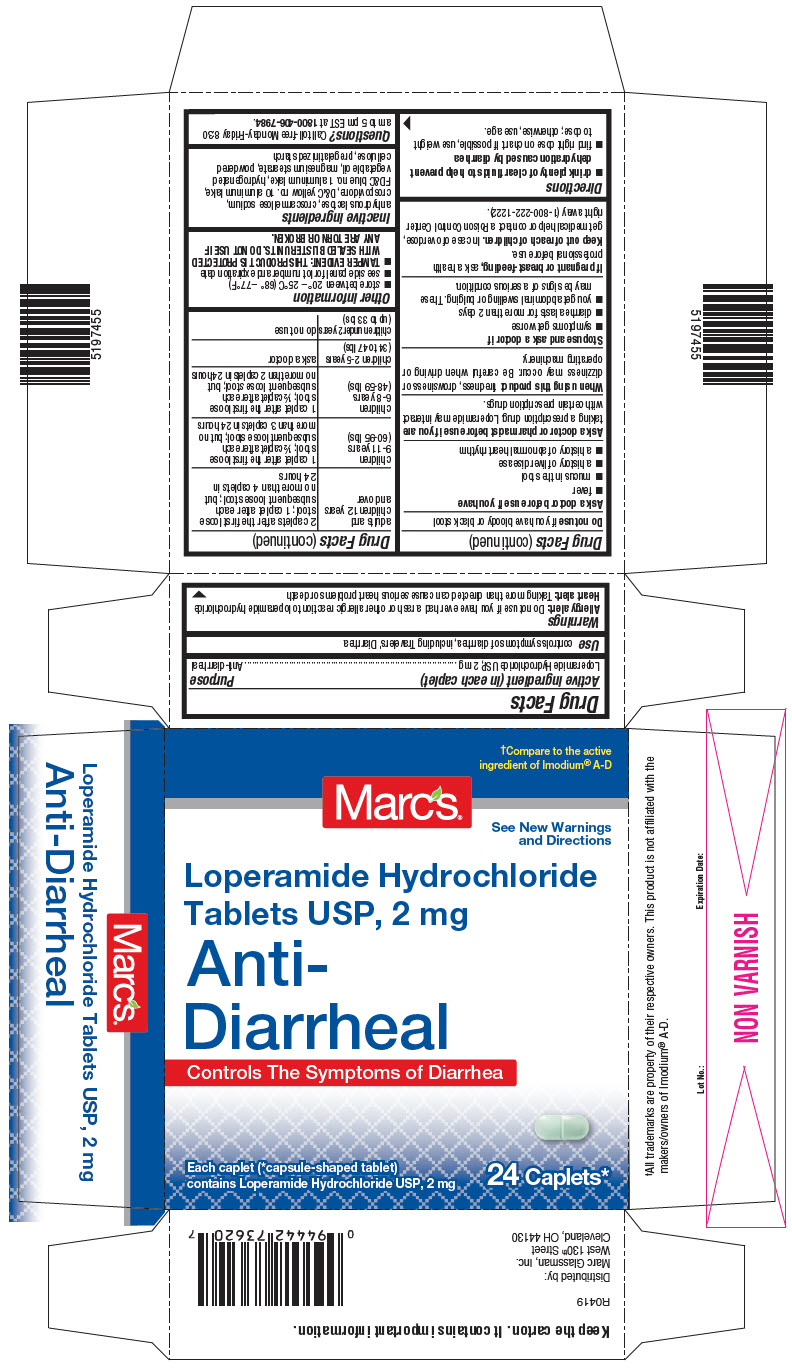
Warnings
Allergy alert
Do not use if you have ever had a rash or other allergic reaction to loperamide hydrochloride
Ask a doctor before use if you have
- fever
- mucus in the stool
- a history of liver disease
- a history of abnormal heart rhythm
Ask a doctor or pharmacist before use if you aretaking a prescription drug. Loperamide may interact with certain prescription drugs.
When using this producttiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
| adults and children 12 years and over | 2 caplets after the first loose stool; 1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hours |
| children 9-11 years (60-95 lbs) | 1 caplet after the first loose stool; ½ caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours |
| children 6-8 years (48-59 lbs) | 1 caplet after the first loose stool; ½ caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours |
| children 2-5 years (34 to 47 lbs) | ask a doctor |
| children under 2 years (up to 33 lbs) | do not use |
Other information
- store between 20° – 25°C (68° – 77°F)
- see side panel for lot number and expiration date
- TAMPER EVIDENT: THIS PRODUCT IS PROTECTED WITH SEALED BLISTER UNITS. DO NOT USE IF ANY ARE TORN OR BROKEN.
Inactive ingredients
anhydrous lactose, croscarmellose sodium, crospovidone, D&C yellow no. 10 aluminum lake, FD&C blue no. 1 aluminum lake, hydrogenated vegetable oil, magnesium stearate, powdered cellulose, pregelatinized starch
PRINCIPAL DISPLAY PANEL - 24 Caplet Blister Pack Carton
†Compare to the active
ingredient of Imodium
®A-D
Marc's ®
See New Warnings
and Directions
Loperamide Hydrochloride
Tablets USP, 2 mg
Anti-
Diarrheal
Controls The Symptoms of Diarrhea
Each caplet (*capsule-shaped tablet)
contains Loperamide Hydrochloride USP, 2 mg
24 Caplets*