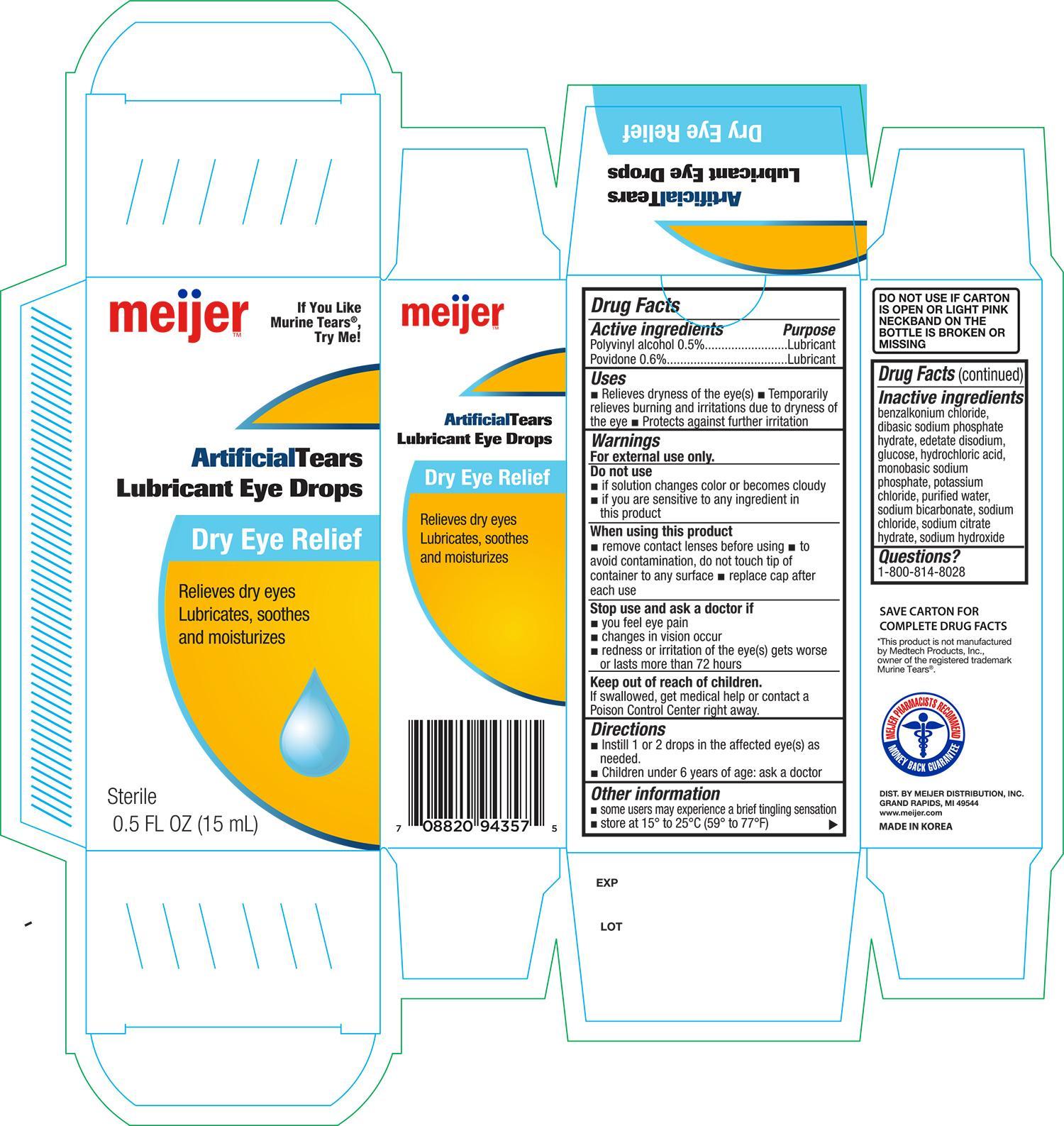
Active ingredients Purpose
Polyvinyl alcohol 0.5%...................................................... Lubricant
Povidone 0.6%................................................................. Lubricant
Uses
- Relieves dryness of the eye(s)
- Temporarily relieves burning and irritations due to dryness of the eye
- Protects against further irritation
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- remove contact lenses before using
- to avoid contamination, do not touch tip of container to any surface
- replace cap after each use
Stop use and ask a doctor if
- you feel eye pain
- changes in vision occur
- redness or irritation of the eye(s) gets worse or lasts more than 72 hours
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Directions
- Instill 1 or 2 drops in the affected eye(s) as needed
- Children under 6 years of age: ask a doctor
Other information
- some users may experience a brief tingling sensation
- store at 15° to 25°C (59° to 77°F)