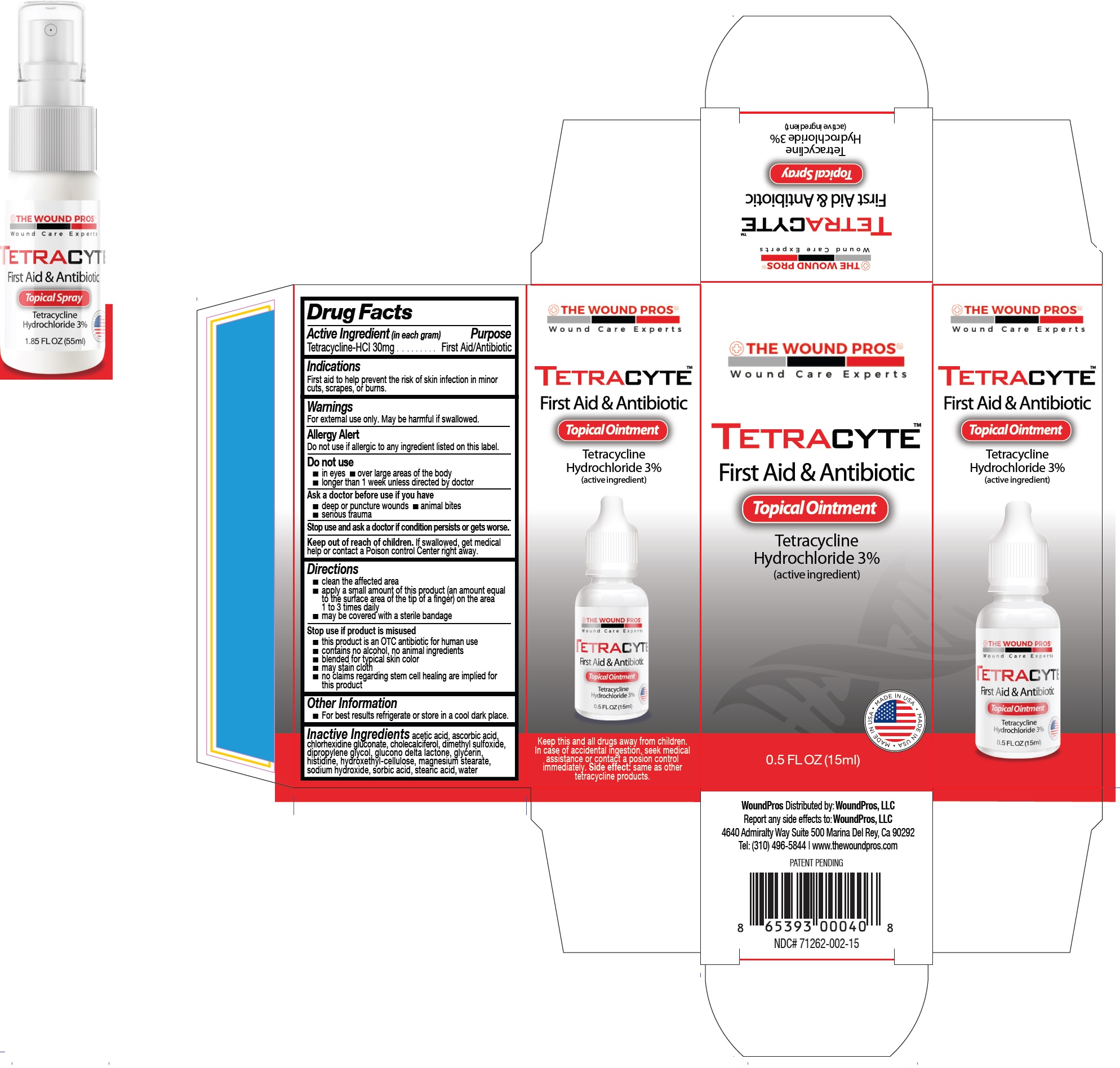
Active Ingredient (in each gram)
Tetracycline-HCL 30mg
Purpose
First Aid/Antibiotic
Indications
First aid to help prevent the risk of skin infection in minor cuts, scrapes, or burns.
Warnings
For external use only. May be harmful if swallowed.
Do not use if allergic to any ingredient listed on this label.
Allergy Alert
Do not use
- in eyes
- over large areas of the body
- longer than 1 week unless directed by doctor
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious trauma
Stop use and ask a doctor
if condition persists or gets worse.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison control Center right away.
Directions
- clean the affected area
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Stop use if product is misused
- this product is an OTC antibiotic for human use
- contains no alcohol, no animal ingredients
- blended for typical skin color
- may stain cloth
- no claims regarding stem cell healing are implied for this product
Other Information
- For best results refrigerate or store in a cool dark place.
Inactive Ingredients
acetic acid, ascorbic acid, chlorhexidine gluconate, cholecalciferol, dimethyl sulfoxide, dipropylene glycol, glucono delta lactone, glycerin, histidine, hydroxethyl-cellulose, magnesium stearate, sodium hydroxide, sorbic acid, stearic acid, water
Package Labeling: