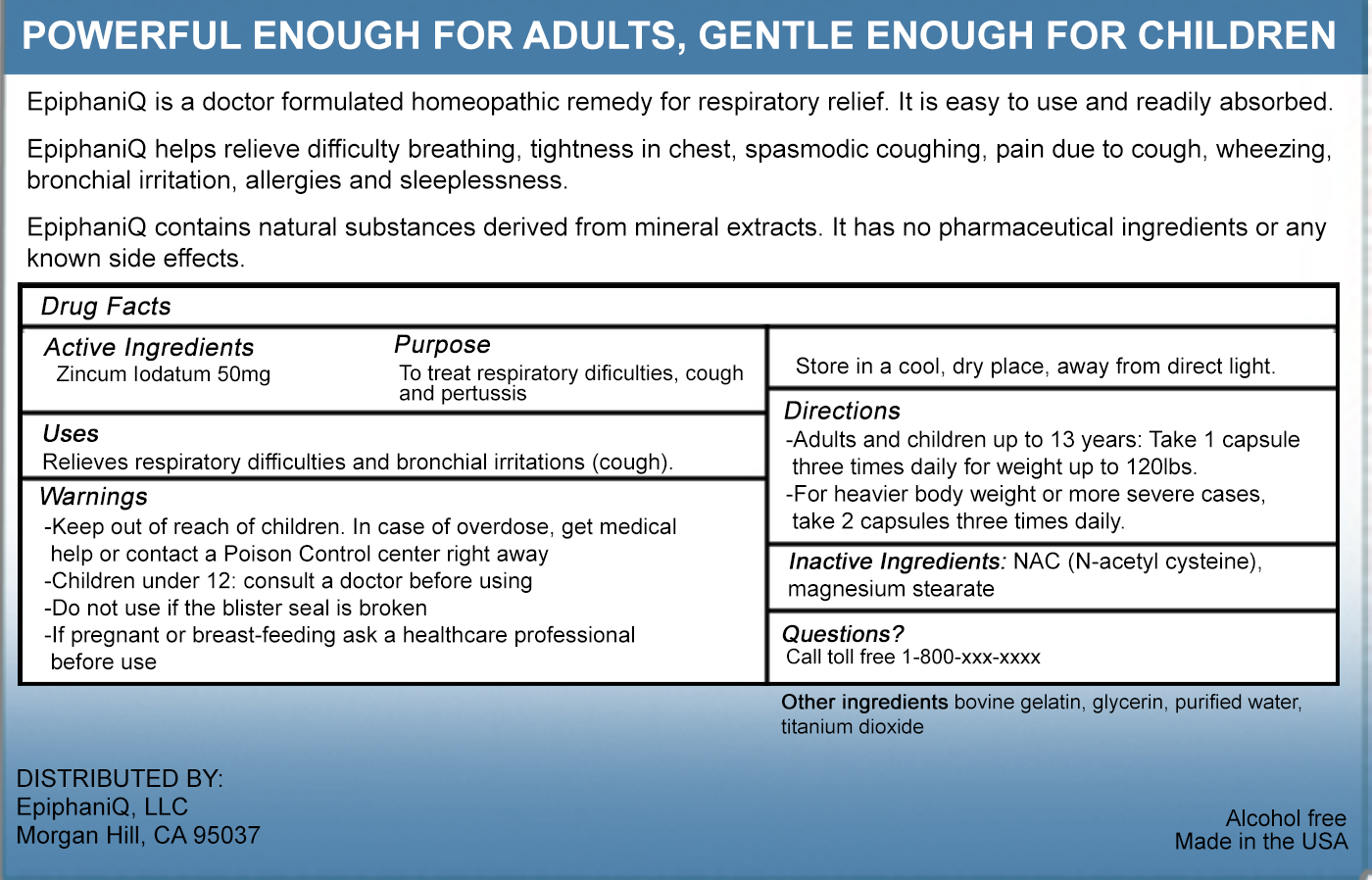
EPIPHANIQ- zincum iodatum capsule
EpiphaniQ LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENT
ZINCUM IODATUM 50 MG
PURPOSE
TO TREAT RESPIRATORY DIFFICULTIES, COUGH AND PERTUSSIS
USES
Relieves respiratory difficulties and bronchial irritations (cough).
WARNINGS
- Children under 12: consult a doctor before using
- Do not use if the blister seal is broken
- If pregnant or breast-feeding ask a healthcare professional before use
- Store in a cool, dry place, away from direct light.
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control center right away
DIRECTIONS
- Adults and children up to 13 years: Take 1 capsule three times daily for weight up to 120lbs.
- For heavier body weight or more severe cases, take 2 capsules three times
INACTIVE INGREDIENTS
NAC (N-acetyl cysteine), magnesium stearate
OTHER INGREDIENTS: BOVINE GELATIN, GLYCERIN, PURIFIED WATER, TITANIUM DIOXIDE
QUESTIONS
CALL TOLL FREE 1-800-XXX-XXXX