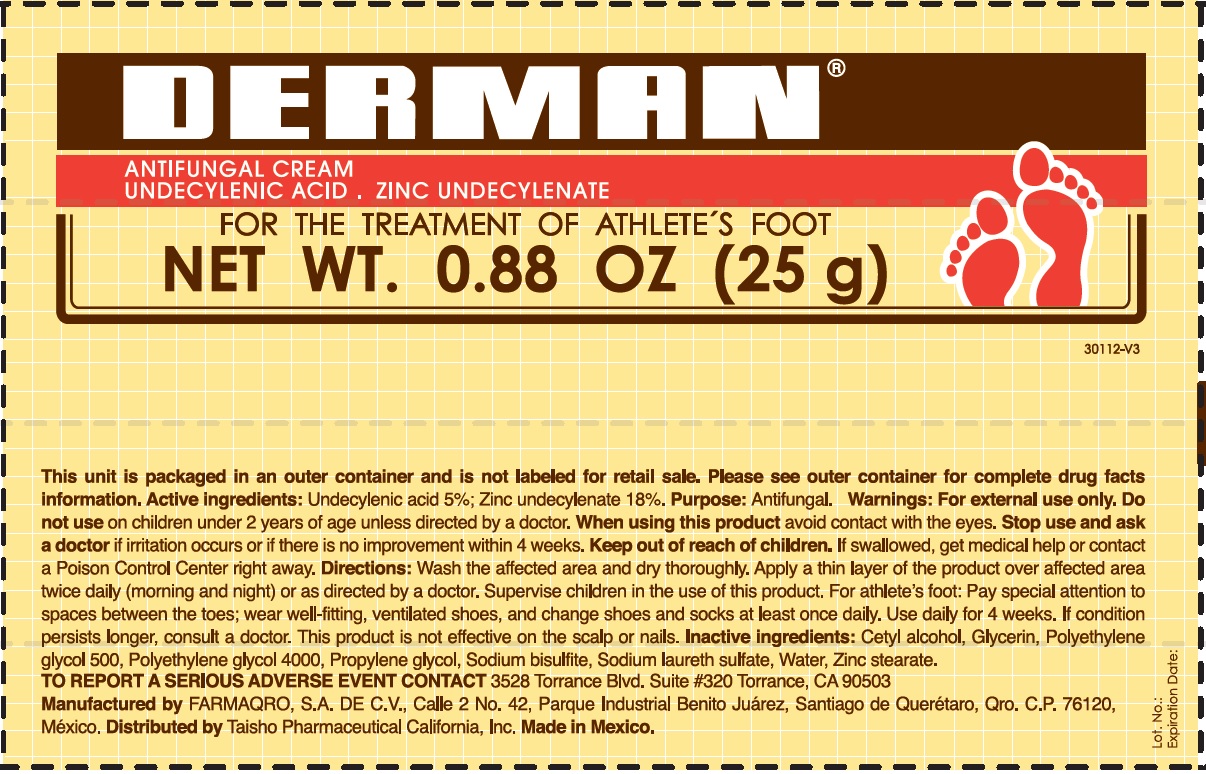
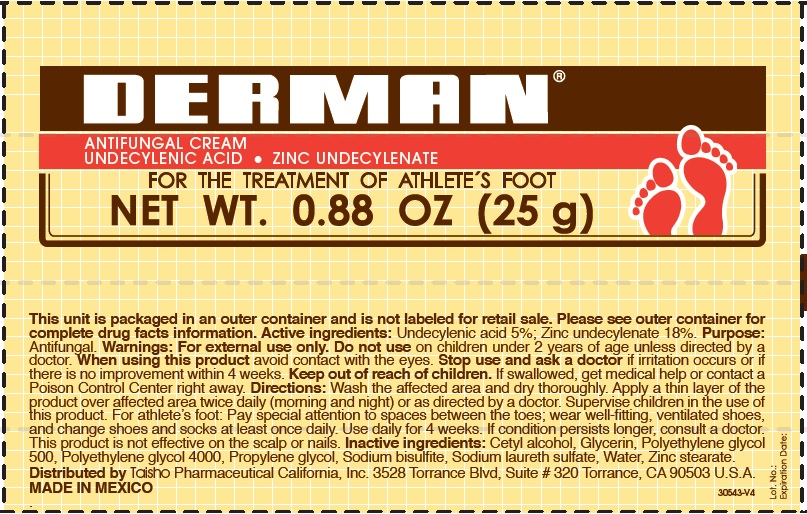
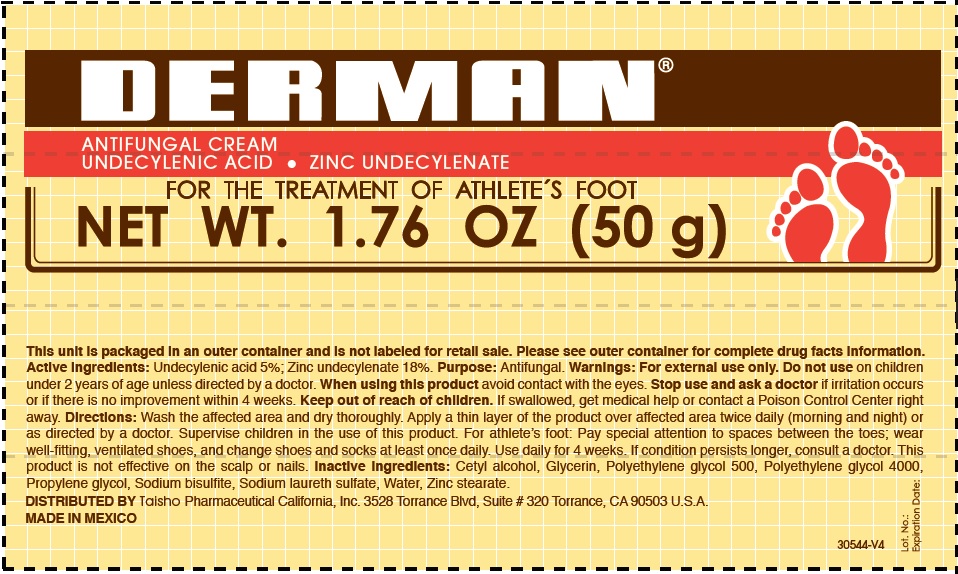
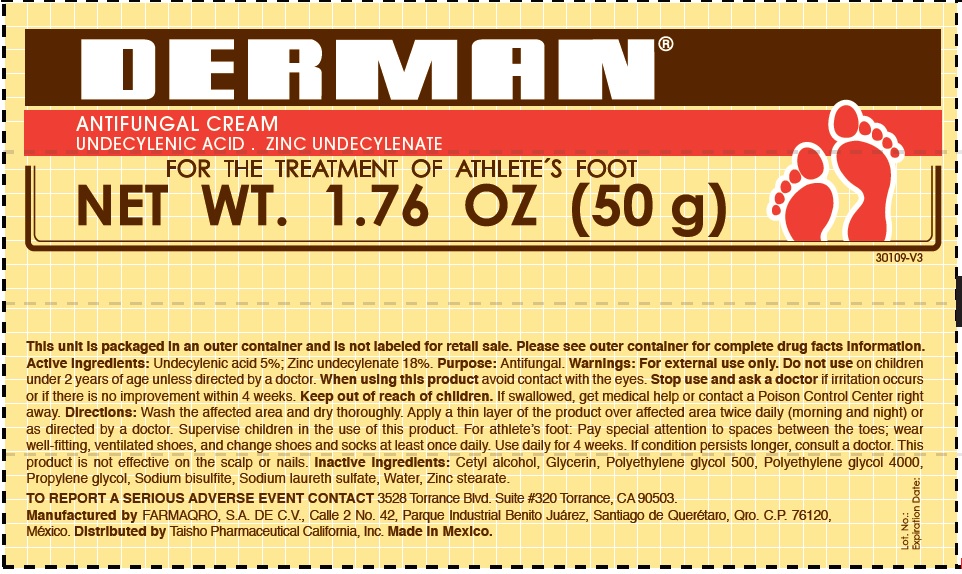
Active Ingredients.
Zinc undecylenate 18%
Undecylenic acid 5%
Uses
For the treatment of athlete´s foot.
Warnings
For external use only
Do not use
on children under 2 years of age, unless directed by a doctor.
When using this product
Avoid contact with the eyes.
Stop use and ask a doctor
if irritation occurs or if there is no improvement within 4 weeks.
Keep out of the reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wash the affected area and dry thoroughly.
- Apply a thin layer of the product over the affected area twice daily (morning and night) or as directed by a doctor.
- Supervise children in the use of this product.
- For athlete's foot: Pay special attention to spaces between the toes; wear well fitting, ventilated shoes, and change shoes and socks at least once daily.
- Use daily for 4 weeks.
- If condition persists, consults a physician.
- This product is not effective on the scalp or nails.
Inactive ingredients
Cetyl alcohol, Glycerin, Polyethylene Glycol 500, Polyethylene glycol 4000, Propylene glycol, Sodium bisulfite, Sodium laureth sulfate, water, Zinc stearate.
Compania Internacional de Comercio, S.A.P.I de C.V.