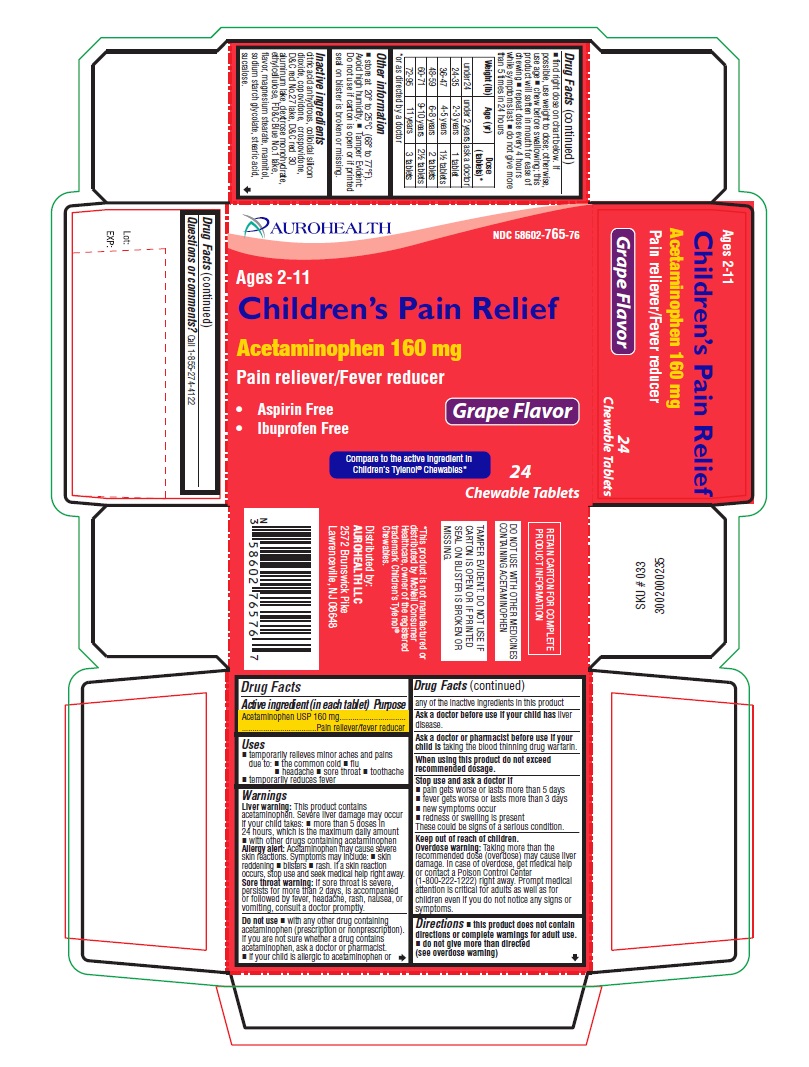
Uses
- temporarily relieves minor aches and pains due to:
- the common cold
- flu
- headache
- sore throat
- toothache
- temporarily reduces fever
Warnings
-
Liver warning:This product contains acetaminophen. Severe liver damage may occur if your child takes:
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
-
Allergy alert:Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash.
If a skin reaction occurs, stop use and seek medical help right away.
- Sore throat warning:If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product.
Stop use and ask a doctor if
- pain gets worse or lasts more than 5 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning:Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- this product does not contain directions or complete warnings for adult use.
- do not give more than directed (see overdose warning)
- find right dose on chart below. If possible, use weight to dose; otherwise, use age
- chew before swallowing; this product will soften in mouth for ease of chewing
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
| Weight (lb)
| Age (yr)
| Dose (tablets)*
|
|---|---|---|
| * or as directed by a doctor | ||
| under 24
| under 2 years
| ask a doctor
|
| 24-35
| 2-3 years
| 1 tablet
|
| 36-47
| 4-5 years
| 1½ tablets
|
| 48-59
| 6-8 years
| 2 tablets
|
| 60-71
| 9-10 years
| 2½ tablets
|
| 72-95
| 11 years
| 3 tablets
|
Other information
- store at 20° to 25°C (68° to 77°F). Avoid high humidity.
- Tamper Evident: Do not use if carton is open or if printed seal on blister is broken or missing.
Inactive ingredients
citric acid anhydrous, colloidal silicon dioxide, copovidone, crospovidone, D&C red No.27 lake, D&C red 30 aluminum lake, dextrose monohydrate, ethylcellulose, FD &C Blue No.1 lake, flavor, magnesium stearate, mannitol, sodium starch glycolate, stearic acid, sucralose.
Questions or comments?
Call 1-855-274-4122
* This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Children's Tylenol
®Chewables.
DO NOT USE WITH OTHER MEDICINES CONTAINING ACETAMINOPHEN
Distributed by:
AUROHEALTH LLC
2572 Brunswick Pike
Lawrenceville, NJ 08648
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 160 mg (24 Chewable Tablets)
AUROHEALTH
NDC 58602-765-76
Ages 2-11
Children's Pain Relief
Acetaminophen 160 mg
Pain reliever/Fever reducer
- Aspirin Free
- Ibuprofen Free
Grape Flavor
Compare to the active ingredient in
Children's Tylenol® Chewables*
24
Chewable Tablets