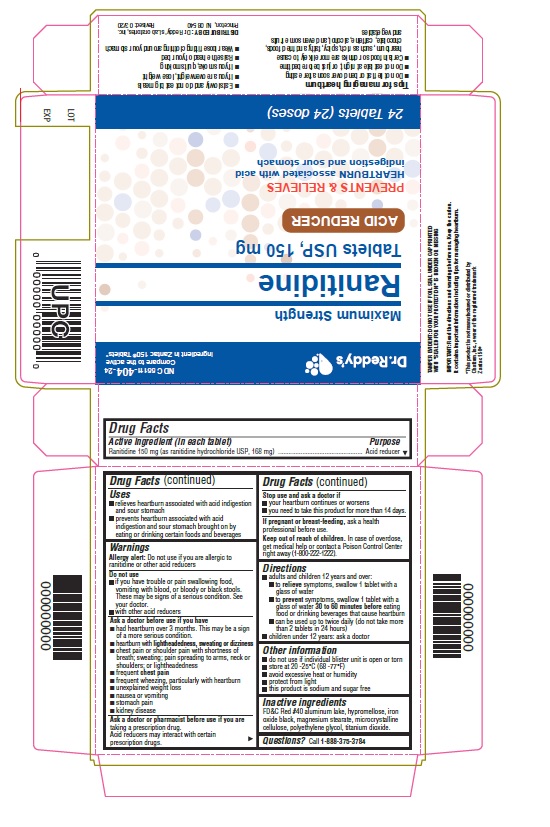
Use(s)
- relieves heartburn associated with acid indigestion and sour stomach
- prevents heartburn associated with acid indigestion and sour stomach brought on by eating or drinking certain foods and beverages
Warnings
Allergy alert: Do not use if you are allergic to ranitidine or other acid reducers
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools.These may be signs of a serious condition. See your doctor.
- with other acid reducers
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- kidney disease
Ask a doctor or pharmacist before use if you are
• taking a prescription drug. Acid reducers may interact with certain prescription drugs.
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water
- to prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn
- can be used up to twice daily (do not take more than 2 tablets in 24 hours)
- children under 12 years: ask a doctor
Other information
- do not use if printed foil under bottle cap is open or torn
- store at 20°-25°C (68°-77°F)
- avoid excessive heat or humidity
- protect from light
- this product is sodium and sugar free
Inactive ingredients
FD&C red #40 aluminum lake, hypromellose, iron oxide black, magnesium stearate, microcrystalline cellulose, polyethylene glycol, titanium dioxide.
Questions?
Call 1-888-375-3784
Read the directions and warnings before use. Keep the carton. It contains important information including tips for managing heartburn.
Tips for managing heartburn
- Do not lie flat or bend over soon after eating
-
Do not eat late at night, or just before bedtime
-
Certain foods or drinks are more likely to cause heartburn, such as rich, spicy, fatty, and fried foods, chocolate, caffeine, alcohol, and even some fruits and vegetables.
-
Eat slowly and do not eat beig meals
-
If you are overweight, lose weight
-
If you smoke, quit smoking
-
Raise the head of your bed
-
Wear loose fitting clothing around your stomach.
Revised: 03/20
PACKAGE LABEL PRINCIPAL DISPLAY PANEL
Container Label:
Dr.Reddy's
NDC 55111-404-34
Maximum Strength
Ranitidine
Tablets USP,150 mg
ACID REDUCER
PREVENTS & RELIEVES
HEARTBURN associated with acid indigestion
and sour stomach
24 Tablets(24 doses)