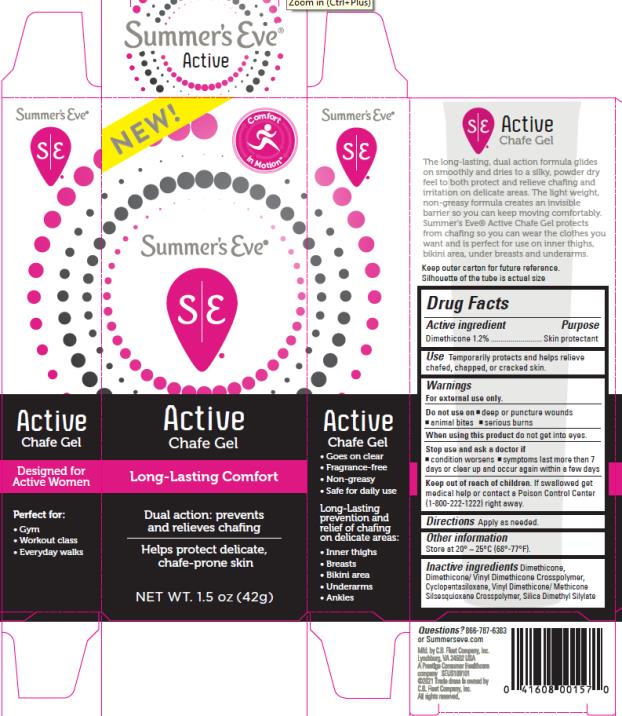
SUMMERS EVE ACTIVE CHAFE- dimethicone gel
C.B. Fleet Company, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Purpose
Dimethicone 1.2%...................................Skin Protectant
Use
Temporarily protects and helps relieve chafed, chapped, or cracked skin
Warnings
For external use only.
Do not use on
- deep or puncture wounds
- animal bites
- serious burns
When using this product
do not get into eyes
Stop use and ask a doctor if
- condition worsens
- symptoms lasts more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
Apply as needed.
Other information
Store at 20° – 25°C (68°-77°F)
Inactive Ingredients
dimethicone, dimethicone/vinyl dimethicone crosspolymer, cyclopentasiloxane, vinyl dimethicone/methicone silsesquioxane crosspolymer, silica dimethyl silylate
Questions?
866 – 787 – 6383 or Summerseve.com
PRINCIPAL DISPLAY PANEL
Summer’s Eve Active Chafe Gel
Dimethicone 1.2%/Skin Protectant
Net Wt. 1.5 oz (42g)
C.B. Fleet Company, Inc.