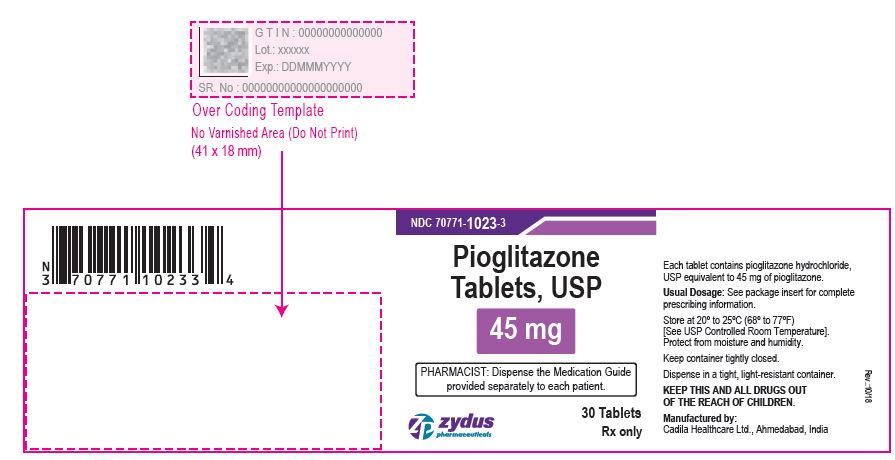
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 70771-1021-3 in bottle of 30 tablets
Pioglitazone Tablets USP, 15 mg
Rx only
30 tablets
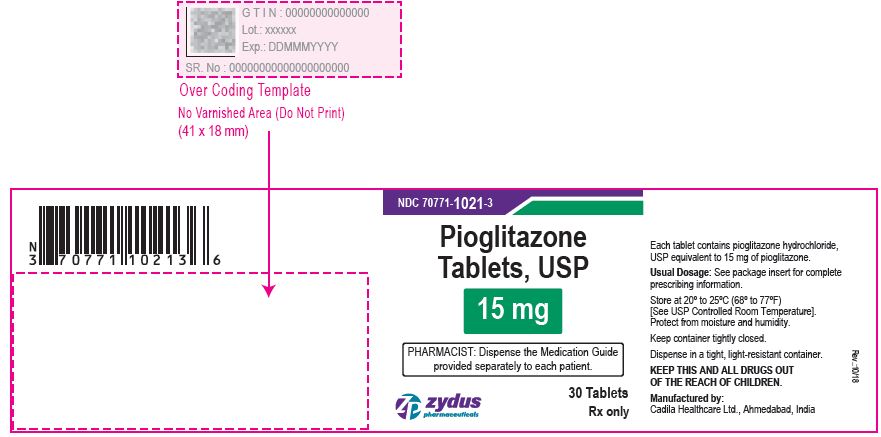
NDC 70771-1022-3 in bottle of 30 tablets
Pioglitazone Tablets USP, 30 mg
Rx only
30 tablets
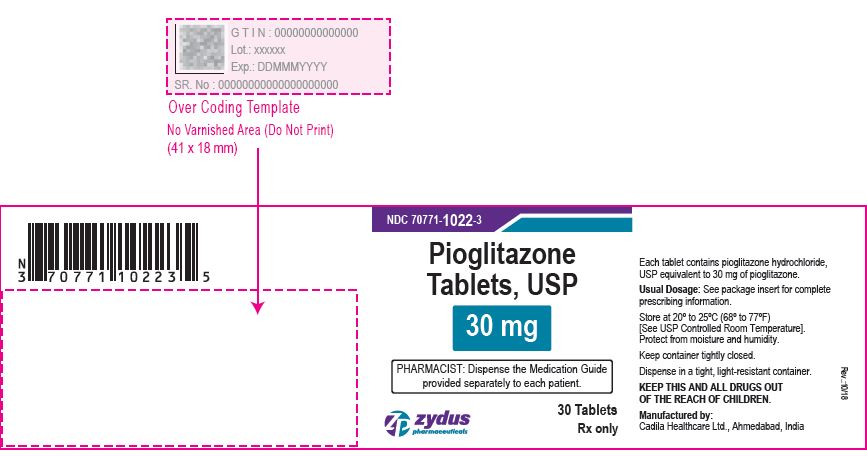
NDC 70771-1023-3 in bottle of 30 tablets
Pioglitazone Tablets USP, 45 mg
Rx only
30 tablets