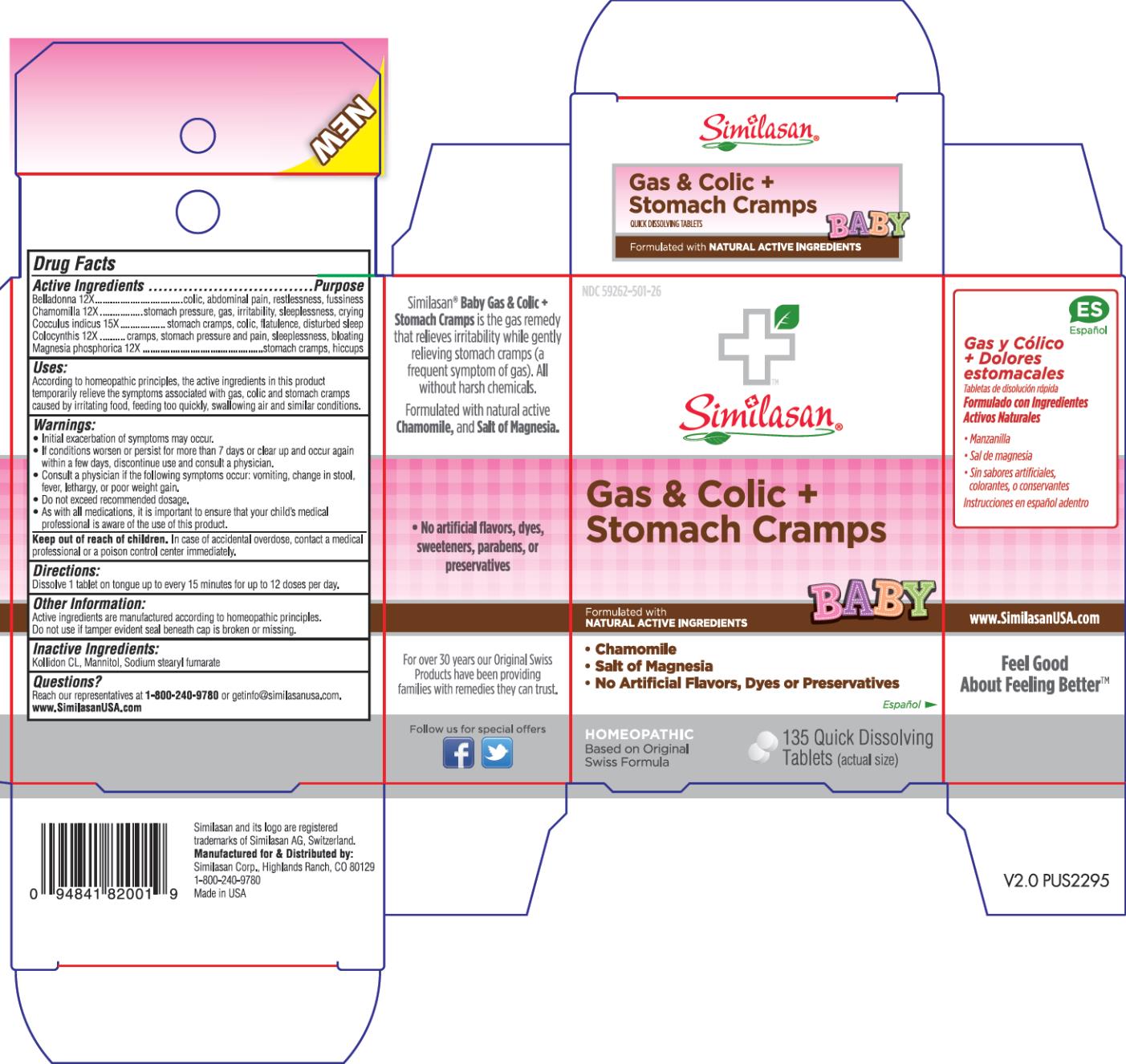
BABY GAS AND COLIC PLUS STOMACH CRAMPS- atropa belladonna, matricaria recutita, anamirta cocculus whole, citrullus colocynthis fruit pulp and magnesium phosphate, dibasic trihydrate tablet, orally disintegrating
Similasan Corporation
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Baby Gas and Colic Plus Stomach Cramps
Uses:
According to homeopathic principles, the active ingredients in this product temporarily relieve the symptoms associated with gas and colic caused by irritating food, feeding too quickly, swallowing air and similar conditions such as:
- gas discomfort
- colic
- stomach cramps
- stomach pressure
- hiccups
- crying
- irritability
Warnings:
- Initial exacerbation of symptoms may occur.
- If conditions worsen or persist for more than 7 days or clear up and occur again within a few days, discontinue use and consult a physician.
- Consulta a physician if the following symptoms occur: vomiting, change in stool, fever, lethargy, or poor weight gain.
- Do not exceed recommended dosage.
- As with all medications, it is important to ensure that your child’s medical professional is aware of the use of this product.
Keep out of reach of children.
In case of accidental overdose, contact a medical professional or a poison control center immediately.
Other Information:
Active ingredients are manufactured according to homeopathic principles.
Do not use if tamper evident seal beneath cap is broken or missing.
| BABY GAS AND COLIC PLUS STOMACH CRAMPS
atropa belladonna, matricaria recutita, anamirta cocculus whole, citrullus colocynthis fruit pulp and magnesium phosphate, dibasic trihydrate tablet, orally disintegrating |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Similasan Corporation (111566530) |