D.H.E. 45- dihydroergotamine mesylate injection, solution
Bausch Health US, LLC
----------
D.H.E. 45®
(dihydroergotamine mesylate)
Injection, USP
DESCRIPTION
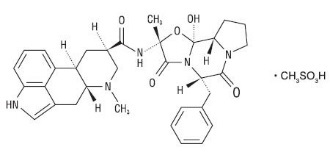
D.H.E. 45 is ergotamine hydrogenated in the 9, 10 position as the mesylate salt. D.H.E. 45 is known chemically as ergotaman-3´,6´,18-trione,9,10-dihydro-12´-hydroxy-2´-methyl-5´-(phenylmethyl)-,(5´α)-, monomethanesulfonate. Its molecular weight is 679.80 and its empirical formula is C33H37N5O5•CH4O3S.
The chemical structure is:
Dihydroergotamine mesylate
C33H37N5O5•CH4O3S Mol. Wt. 679.80
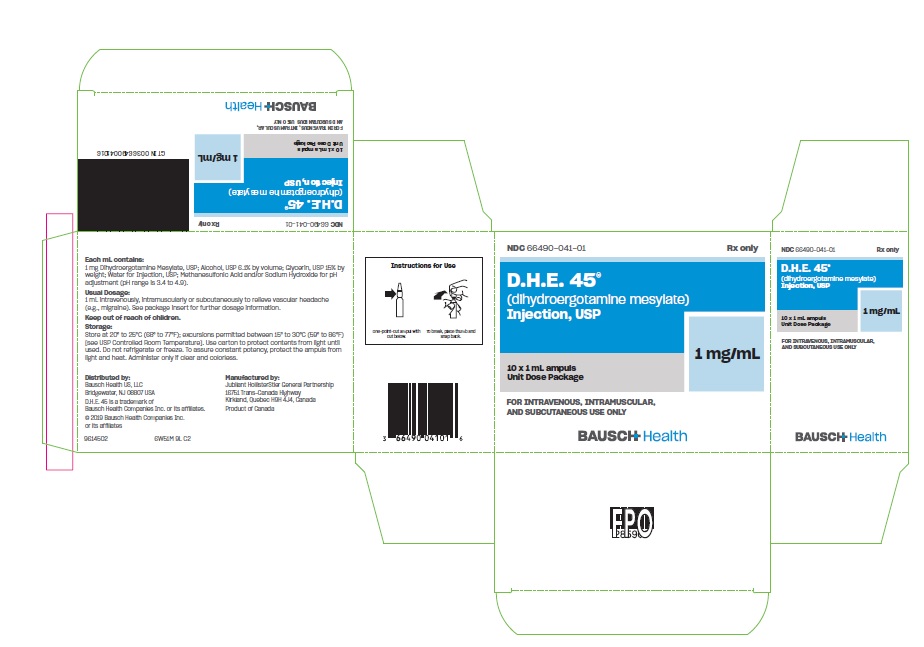
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP is a clear, colorless solution supplied in sterile ampuls for intravenous, intramuscular, or subcutaneous administration. Each mL contains 1 mg Dihydroergotamine Mesylate, USP; Alcohol, USP 6.1% by volume; Glycerin, USP 15% by weight; Water for Injection, USP; Methanesulfonic Acid and/or Sodium Hydroxide for pH adjustment (pH range is 3.4 to 4.9).
CLINICAL PHARMACOLOGY
Mechanism of Action
Dihydroergotamine binds with high affinity to 5-HT1Dα and 5-HT1Dβ receptors. It also binds with high affinity to serotonin 5-HT1A, 5-HT2A, and 5-HT2C receptors, noradrenaline α2A, α2B and α1 receptors, and dopamine D2L and D3 receptors.
The therapeutic activity of dihydroergotamine in migraine is generally attributed to the agonist effect at 5-HT1D receptors. Two current theories have been proposed to explain the efficacy of 5-HT1D receptor agonists in migraine. One theory suggests that activation of 5-HT1D receptors located on intracranial blood vessels, including those on arteriovenous anastomoses, leads to vasoconstriction, which correlates with the relief of migraine headache. The alternative hypothesis suggests that activation of 5-HT1D receptors on sensory nerve endings of the trigeminal system results in the inhibition of pro-inflammatory neuropeptide release.
In addition, dihydroergotamine possesses oxytocic properties.
Pharmacokinetics
Absorption
Absolute bioavailability for the subcutaneous and intramuscular route have not been determined; however, no difference was observed in dihydroergotamine bioavailability from intramuscular and subcutaneous doses.
Dihydroergotamine mesylate is poorly bioavailable following oral administration.
Distribution
Dihydroergotamine mesylate is 93% plasma protein bound. The apparent steady-state volume of distribution is approximately 800 liters.
Metabolism
Four dihydroergotamine mesylate metabolites have been identified in human plasma following oral administration. The major metabolite, 8´-β-hydroxydihydroergotamine, exhibits affinity equivalent to its parent for adrenergic and 5-HT receptors and demonstrates equivalent potency in several venoconstrictor activity models, in vivo and in vitro. The other metabolites, (i.e., dihydrolysergic acid, dihydrolysergic amide) and a metabolite formed by oxidative opening of the proline ring are of minor importance. Following nasal administration, total metabolites represent only 20% to 30% of plasma AUC. Quantitative pharmacokinetic characterization of the four metabolites has not been performed.
Excretion
The major excretory route of dihydroergotamine is via the bile in the feces. The total body clearance is 1.5 L/min which reflects mainly hepatic clearance. Only 6% to 7% of unchanged dihydroergotamine is excreted in the urine after intramuscular injection. The renal clearance (0.1 L/min) is unaffected by the route of dihydroergotamine administration. The decline of plasma dihydroergotamine after intramuscular or intravenous administration is multi-exponential with a terminal half-life of about 9 hours.
Subpopulations
No studies have been conducted on the effect of renal or hepatic impairment, gender, race, or ethnicity on dihydroergotamine pharmacokinetics. D.H.E. 45 (dihydroergotamine mesylate) Injection, USP is contraindicated in patients with severely impaired hepatic or renal function (see CONTRAINDICATIONS).
Interactions
Pharmacokinetic interactions have been reported in patients treated orally with other ergot alkaloids (e.g., increased levels of ergotamine) and macrolide antibiotics, principally troleandomycin, presumably due to inhibition of cytochrome P4503A metabolism of the alkaloids by troleandomycin. Dihydroergotamine has also been shown to be an inhibitor of cytochrome P4503A catalyzed reactions and rare reports of ergotism have been obtained from patients treated with dihydroergotamine and macrolide antibiotics (e.g., troleandomycin, clarithromycin, erythromycin), and in patients treated with dihydroergotamine and protease inhibitors (e.g., ritonavir), presumably due to inhibition of cytochrome P4503A metabolism of ergotamine (see CONTRAINDICATIONS). No pharmacokinetic interactions involving other cytochrome P450 isoenzymes are known.
INDICATIONS AND USAGE
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP is indicated for the acute treatment of migraine headaches with or without aura and the acute treatment of cluster headache episodes.
CONTRAINDICATIONS
There have been a few reports of serious adverse events associated with the coadministration of dihydroergotamine and potent CYP3A4 inhibitors, such as protease inhibitors and macrolide antibiotics, resulting in vasospasm that led to cerebral ischemia and/or ischemia of the extremities. The use of potent CYP3A4 inhibitors (i.e., ritonavir, nelfinavir, indinavir, erythromycin, clarithromycin, troleandomycin, ketoconazole, itraconazole) with dihydroergotamine is, therefore, contraindicated (see WARNINGS, CYP3A4 Inhibitors).
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP should not be given to patients with ischemic heart disease (e.g., angina pectoris, history of myocardial infarction, or documented silent ischemia) or to patients who have clinical symptoms or findings consistent with coronary artery vasospasm including Prinzmetal's variant angina (see WARNINGS).
Because D.H.E. 45 (dihydroergotamine mesylate) Injection, USP may increase blood pressure, it should not be given to patients with uncontrolled hypertension.
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP, 5-HT1 agonists (e.g., sumatriptan), ergotamine-containing or ergot-type medications or methysergide should not be used within 24 hours of each other.
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP should not be administered to patients with hemiplegic or basilar migraine.
In addition to those conditions mentioned above, D.H.E. 45 (dihydroergotamine mesylate) Injection, USP is also contraindicated in patients with known peripheral arterial disease, sepsis, following vascular surgery and severely impaired hepatic or renal function.
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP is contraindicated in patients who have previously shown hypersensitivity to ergot alkaloids.
Dihydroergotamine mesylate should not be used with peripheral and central vasoconstrictors because the combination may result in additive or synergistic elevation of blood pressure.
WARNINGS
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP should only be used where a clear diagnosis of migraine headache has been established.
CYP3A4 Inhibitors (e.g., Macrolide Antibiotics and Protease Inhibitors)
There have been rare reports of serious adverse events in connection with the coadministration of dihydroergotamine and potent CYP3A4 inhibitors, such as protease inhibitors and macrolide antibiotics, resulting in vasospasm that led to cerebral ischemia and/or and ischemia of the extremities. The use of potent CYP3A4 inhibitors with dihydroergotamine should therefore be avoided (see CONTRAINDICATIONS). Examples of some of the more potent CYP3A4 inhibitors include: antifungals ketoconazole and itraconazole, the protease inhibitors ritonavir, nelfinavir, and indinavir, and macrolide antibiotics erythromycin, clarithromycin, and troleandomycin. Other less potent CYP3A4 inhibitors should be administered with caution. Less potent inhibitors include saquinavir, nefazodone, fluconazole, grapefruit juice, fluoxetine, fluvoxamine, zileuton, and clotrimazole. These lists are not exhaustive, and the prescriber should consider the effects on CYP3A4 of other agents being considered for concomitant use with dihydroergotamine.
Fibrotic Complications
There have been reports of pleural and retroperitoneal fibrosis in patients following prolonged daily use of injectable dihydroergotamine mesylate. Rarely, prolonged daily use of other ergot alkaloid drugs has been associated with cardiac valvular fibrosis. Rare cases have also been reported in association with the use of injectable dihydroergotamine mesylate; however, in those cases, patients also received drugs known to be associated with cardiac valvular fibrosis.
Administration of D.H.E. 45 (dihydroergotamine mesylate) Injection, USP, should not exceed the dosing guidelines and should not be used for chronic daily administration (see DOSAGE AND ADMINISTRATION).
Risk of Myocardial Ischemia and/or Infarction and Other Adverse Cardiac Events
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP should not be used by patients with documented ischemic or vasospastic coronary artery disease (see CONTRAINDICATIONS). It is strongly recommended that D.H.E. 45 (dihydroergotamine mesylate) Injection, USP not be given to patients in whom unrecognized coronary artery disease (CAD) is predicted by the presence of risk factors (e.g., hypertension, hypercholesterolemia, smoker, obesity, diabetes, strong family history of CAD, females who are surgically or physiologically postmenopausal, or males who are over 40 years of age) unless a cardiovascular evaluation provides satisfactory clinical evidence that the patient is reasonably free of coronary artery and ischemic myocardial disease or other significant underlying cardiovascular disease. The sensitivity of cardiac diagnostic procedures to detect cardiovascular disease or predisposition to coronary artery vasospasm is modest, at best. If, during the cardiovascular evaluation, the patient's medical history or electrocardiographic investigations reveal findings indicative of or consistent with coronary artery vasospasm or myocardial ischemia, D.H.E. 45 (dihydroergotamine mesylate) Injection, USP should not be administered (see CONTRAINDICATIONS).
For patients with risk factors predictive of CAD who are determined to have a satisfactory cardiovascular evaluation, it is strongly recommended that administration of the first dose of D.H.E. 45 (dihydroergotamine mesylate)
Injection, USP take place in the setting of a physician's office or similar medically staffed and equipped facility unless the patient has previously received dihydroergotamine mesylate. Because cardiac ischemia can occur in the absence of clinical symptoms, consideration should be given to obtaining on the first occasion of use, an electrocardiogram (ECG) during the interval immediately following D.H.E. 45 (dihydroergotamine mesylate) Injection, USP in those patients with risk factors.
It is recommended that patients who are intermittent long-term users of D.H.E. 45 (dihydroergotamine mesylate) Injection, USP and who have or acquire risk factors predictive of CAD, as described above, undergo periodic interval cardiovascular evaluation as they continue to use D.H.E. 45 (dihydroergotamine mesylate) Injection, USP.
The systematic approach described above is currently recommended as a method to identify patients in whom D.H.E. 45 (dihydroergotamine mesylate) Injection, USP may be used to treat migraine headaches with an acceptable margin of cardiovascular safety.
Cardiac Events and Fatalities
The potential for adverse cardiac events exists. Serious adverse cardiac events, including acute myocardial infarction, life-threatening disturbances of cardiac rhythm, and death have been reported to have occurred following the administration of dihydroergotamine mesylate injection. Considering the extent of use of dihydroergotamine mesylate in patients with migraine, the incidence of these events is extremely low.
Drug-Associated Cerebrovascular Events and Fatalities
Cerebral hemorrhage, subarachnoid hemorrhage, stroke, and other cerebrovascular events have been reported in patients treated with D.H.E. 45 (dihydroergotamine mesylate) Injection, USP; and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the D.H.E. 45 (dihydroergotamine mesylate) Injection, USP having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine, when they were not. It should be noted that patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, transient ischemic attack).
Other Vasospasm-Related Events
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP, like other ergot alkaloids, may cause vasospastic reactions other than coronary artery vasospasm. Myocardial, peripheral vascular, and colonic ischemia have been reported with D.H.E. 45 (dihydroergotamine mesylate) Injection, USP.
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP associated vasospastic phenomena may also cause muscle pains, numbness, coldness, pallor, and cyanosis of the digits. In patients with compromised circulation, persistent vasospasm may result in gangrene or death. D.H.E. 45 (dihydroergotamine mesylate) Injection, USP should be discontinued immediately if signs or symptoms of vasoconstriction develop.
Increase in Blood Pressure
Significant elevation in blood pressure has been reported on rare occasions in patients with and without a history of hypertension treated with dihydroergotamine mesylate injection. D.H.E. 45 (dihydroergotamine mesylate) Injection, USP is contraindicated in patients with uncontrolled hypertension (see CONTRAINDICATIONS).
An 18% increase in mean pulmonary artery pressure was seen following dosing with another 5-HT1 agonist in a study evaluating subjects undergoing cardiac catheterization.
Medication Overuse Headache
Overuse of acute migraine drugs (e.g., ergotamines, triptans, opioids, or a combination of these drugs for 10 or more days per month) may lead to exacerbation of headache (i.e., medication overuse headache). Medication overuse headache may present as migraine-like daily headaches or as a marked increase in frequency of migraine attacks. Detoxification of patients including withdrawal of the overused drugs and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary.
Preterm Labor
Based on the mechanism of action of dihydroergotamine and findings from the published literature, D.H.E. 45 may cause preterm labor. Avoid use of D.H.E. 45 during pregnancy (see PRECAUTIONS).
PRECAUTIONS
General
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP may cause coronary artery vasospasm; patients who experience signs or symptoms suggestive of angina following its administration should, therefore, be evaluated for the presence of CAD or a predisposition to variant angina before receiving additional doses. Similarly, patients who experience other symptoms or signs suggestive of decreased arterial flow, such as ischemic bowel syndrome or Raynaud's syndrome following the use of any 5-HT agonist are candidates for further evaluation (see WARNINGS).
Information for Patients
The text of a patient information sheet is printed at the end of this insert. To assure safe and effective use of D.H.E. 45 (dihydroergotamine mesylate) Injection, USP, the information and instructions provided in the patient information sheet should be discussed with patients.
Patients should be advised to report to the physician immediately any of the following: numbness or tingling in the fingers and toes, muscle pain in the arms and legs, weakness in the legs, pain in the chest, temporary speeding or slowing of the heart rate, swelling, or itching.
Prior to the initial use of the product by a patient, the prescriber should take steps to ensure that the patient understands how to use the product as provided (see Patient Information Sheet and product packaging).
Administration of D.H.E. 45 (dihydroergotamine mesylate) Injection, USP should not exceed the dosing guidelines and should not be used for chronic daily administration (see DOSAGE AND ADMINISTRATION).
Drug Interactions
Vasoconstrictors
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP should not be used with peripheral vasoconstrictors because the combination may cause synergistic elevation of blood pressure.
Sumatriptan
Sumatriptan has been reported to cause coronary artery vasospasm, and its effect could be additive with D.H.E. 45 (dihydroergotamine mesylate) Injection, USP. Sumatriptan and D.H.E. 45 (dihydroergotamine mesylate) Injection, USP should not be taken within 24 hours of each other (see CONTRAINDICATIONS).
Beta Blockers
Although the results of a clinical study did not indicate a safety problem associated with the administration of D.H.E. 45 (dihydroergotamine mesylate) Injection, USP to subjects already receiving propranolol, there have been reports that propranolol may potentiate the vasoconstrictive action of ergotamine by blocking the vasodilating property of epinephrine.
Nicotine
Nicotine may provoke vasoconstriction in some patients, predisposing to a greater ischemic response to ergot therapy.
CYP3A4 Inhibitors (e.g., Macrolide Antibiotics and Protease Inhibitors) (see CONTRAINDICATIONS and WARNINGS)
SSRI's
Weakness, hyperreflexia, and incoordination have been reported rarely when 5-HT1 agonists have been co-administered with SSRI's (e.g., fluoxetine, fluvoxamine, paroxetine, sertraline). There have been no reported cases from spontaneous reports of drug interaction between SSRI's and D.H.E. 45 (dihydroergotamine mesylate) Injection, USP.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 2-year mouse carcinogenicity study, subcutaneous (SC) administration of dihydroergotamine mesylate (0, 0.5, 1.5 or 5 mg/kg/day) resulted in an increased incidence of fibrosarcoma at the injection sites in males and females at the high dose. The higher dose not associated with an increase in tumors (1.5 mg/kg/day) is approximately 2 times the recommended human dose (RHD) of 3 mg/day SC on a body surface area (mg/m2) basis.
In a 2-year rat carcinogenicity study, intranasal administration of dihydroergotamine mesylate (0, 0.4, 0.8 or 1.6 mg/day for 13 weeks, followed by 0, 0.08, 0.24 or 0.8 mg/day for the remainder of the study) did not result in an increase in tumors.
Mutagenesis
Dihydroergotamine mesylate was clastogenic in two in vitro chromosomal aberration assays, the V79 Chinese hamster cell assay with metabolic activation and the cultured human peripheral blood lymphocyte assay. There was no evidence of mutagenic potential when dihydroergotamine mesylate was tested in the presence or absence of metabolic activation in two gene mutation assays (the Ames test and the in vitro mammalian Chinese hamster V79/HGPRT assay) and in an assay for DNA damage (the rat hepatocyte unscheduled DNA synthesis test). Dihydroergotamine was not clastogenic in the in vivo mouse and hamster micronucleus tests.
Pregnancy
Risk Summary
Available data from published literature indicate an increased risk of preterm delivery
with D.H.E. 45 use during pregnancy. Avoid use of D.H.E. 45 during pregnancy (see WARNINGS). Data collected over decades have shown no increased risk of major birth defects or miscarriage with the use of dihydroergotamine mesylate during pregnancy.
In animal reproduction studies, adverse effects on development were observed following intranasal administration of dihydroergotamine mesylate during pregnancy (decreased fetal body weight and/or skeletal ossification) in rats and rabbits or during pregnancy and lactation in rats (decreased body weight and impaired reproductive function in the offspring) at doses that were not associated with maternal toxicity (see Data).
The estimated rate of major birth defects (2.2% to 2.9%) and miscarriage (17%) among deliveries to women with migraine are similar to rates reported in women without migraine. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Intranasal administration of dihydroergotamine mesylate to pregnant rats throughout the period of organogenesis resulted in decreased fetal body weight and/or skeletal ossification at doses of 0.16 mg/day and greater. A no-effect level for adverse effects on embryofetal development was not identified in rats. Intranasal administration of dihydroergotamine mesylate to pregnant rabbits throughout organogenesis resulted in decreased skeletal ossification at 3.6 mg/day. The no-effect dose for adverse effects on embryofetal development in rabbits was 1.2 mg/day.
Intranasal administration of dihydroergotamine mesylate to female rats throughout pregnancy and lactation resulted in decreased body weight and impaired reproductive function (decreased mating indices) in the offspring at doses of 0.16 mg/day or greater. A no-effect dose for adverse effects on pre- and postnatal development in rats was not established. Effects on offspring development occurred at doses below those that produced evidence of maternal toxicity in these studies.
Dihydroergotamine-induced intrauterine growth retardation has been attributed to reduced uteroplacental blood flow resulting from prolonged vasoconstriction of the uterine vessels and/or increased myometrial tone.
Nursing Mothers
Risk Summary
There are no data on the presence of dihydroergotamine in human milk; however, ergotamine, a related drug, is present in human milk. There are reports of vomiting, diarrhea, weak pulse, and unstable blood pressure in breastfed infants exposed to ergotamine. D.H.E. 45 may reduce milk supply because it may decrease prolactin levels.
Because of the potential for reduced milk supply and serious adverse events in the breastfed infant, including diarrhea, vomiting, weak pulse, and unstable blood pressure, advise patients not to breastfeed during treatment with D.H.E. 45 and for 3 days after the last dose. Breast milk supply during this time should be pumped and discarded.
ADVERSE REACTIONS
Serious cardiac events, including some that have been fatal, have occurred following use of D.H.E. 45 (dihydroergotamine mesylate) Injection, USP, but are extremely rare. Events reported have included coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, and ventricular fibrillation (see CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS). Fibrotic complications have been reported in association with long term use of injectable dihydroergotamine mesylate (see WARNINGS, Fibrotic Complications).
Post-introduction Reports
The following events derived from postmarketing experience have been occasionally reported in patients receiving D.H.E. 45 (dihydroergotamine mesylate) Injection, USP: vasospasm, paraesthesia, hypertension, dizziness, anxiety, dyspnea, headache, flushing, diarrhea, rash, increased sweating, and pleural and retroperitoneal fibrosis after long-term use of dihydroergotamine. Extremely rare cases of myocardial infarction and stroke have been reported. A causal relationship has not been established.
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP is not recommended for prolonged daily use (see DOSAGE AND ADMINISTRATION).
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG ABUSE AND DEPENDENCE
Currently available data have not demonstrated drug abuse or psychological dependence with dihydroergotamine. However, cases of drug abuse and psychological dependence in patients on other forms of ergot therapy have been reported. Thus, due to the chronicity of vascular headaches, it is imperative that patients be advised not to exceed recommended dosages.
OVERDOSAGE
To date, there have been no reports of acute overdosage with this drug. Due to the risk of vascular spasm, exceeding the recommended dosages of D.H.E. 45 (dihydroergotamine mesylate) Injection, USP is to be avoided. Excessive doses of dihydroergotamine may result in peripheral signs and symptoms of ergotism. Treatment includes discontinuance of the drug, local application of warmth to the affected area, the administration of vasodilators, and nursing care to prevent tissue damage.
In general, the symptoms of an acute D.H.E. 45 (dihydroergotamine mesylate) Injection, USP overdose are similar to those of an ergotamine overdose, although there is less pronounced nausea and vomiting with D.H.E. 45 (dihydroergotamine mesylate) Injection, USP. The symptoms of an ergotamine overdose include the following: numbness, tingling, pain, and cyanosis of the extremities associated with diminished or absent peripheral pulses; respiratory depression; an increase and/or decrease in blood pressure, usually in that order; confusion, delirium, convulsions, and coma; and/or some degree of nausea, vomiting, and abdominal pain.
In laboratory animals, significant lethality occurs when dihydroergotamine is given at I.V. doses of 44 mg/kg in mice, 130 mg/kg in rats, and 37 mg/kg in rabbits.
Up-to-date information about the treatment of overdosage can often be obtained from a certified Regional Poison Control Center. Telephone numbers of certified Poison Control Centers are listed in the Physician's Desk Reference (PDR).*
DOSAGE AND ADMINISTRATION
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP should be administered in a dose of 1 mL intravenously, intramuscularly or subcutaneously. The dose can be repeated, as needed, at 1-hour intervals to a total dose of 3 mL for intramuscular or subcutaneous delivery or 2 mL for intravenous delivery in a 24-hour period. The total weekly dosage should not exceed 6 mL. D.H.E. 45 (dihydroergotamine mesylate) Injection, USP should not be used for chronic daily administration.
HOW SUPPLIED/STORAGE AND HANDLING
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP
Available as a clear, colorless, sterile solution in single 1 mL sterile ampuls containing 1 mg of dihydroergotamine mesylate per mL, in packages of 10 (NDC 66490-041-01).
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Use carton to protect contents from light until used.
Do not refrigerate or freeze.
To assure constant potency, protect the ampuls from light and heat. Administer only if clear and colorless.
INSTRUCTION FOR PATIENTS ON SUBCUTANEOUS SELF-INJECTION
Information for the Patient
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP
Before self-injecting D.H.E. 45 (dihydroergotamine mesylate) Injection, USP by subcutaneous administration, you will need to obtain professional instruction on how to properly administer your medication. Below are some of the steps you should follow carefully. Read this leaflet completely before using this medication.
This leaflet does not contain all of the information on D.H.E. 45 (dihydroergotamine mesylate) Injection, USP. Your pharmacist and/or health care provider can provide more detailed information.
Purpose of your medication
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP is intended to treat an active migraine headache. Do not try to use it to prevent a headache if you have no symptoms. Do not use it to treat common tension headache or a headache that is not at all typical of your usual migraine headache. Administration of D.H.E. 45 (dihydroergotamine mesylate) Injection, USP should not exceed the dosing guidelines and should not be used for chronic daily administration. There have been reports of fibrosis (stiffening) in the lung or kidney areas in patients following prolonged daily use of injectable dihydroergotamine mesylate. Rarely, prolonged daily use of other ergot alkaloid drugs (the class of drugs to which D.H.E. 45 (dihydroergotamine mesylate) Injection, USP belongs) has been associated with heart valvular fibrosis.
Rare cases have also been reported in association with the use of injectable dihydroergotamine mesylate; however, in those cases, patients also received drugs known to be associated with heart valvular fibrosis.
Do not use D.H.E. 45 (dihydroergotamine mesylate) Injection, USP if you:
- •
- have any disease affecting your heart, arteries, or circulation.
- •
- are taking certain anti-HIV medications (protease inhibitors).
- •
- are taking a macrolide antibiotic such as troleandomycin, clarithromycin or erythromycin.
Important questions to consider before using
D.H.E. 45 (dihydroergotamine mesylate) Injection, USP
Please answer the following questions before you use your D.H.E. 45 (dihydroergotamine mesylate) Injection, USP.
If you answer YES to any of these questions or are unsure of the answer, you should talk to your doctor before using D.H.E. 45 (dihydroergotamine mesylate) Injection, USP.
- •
- Do you have high blood pressure?
- •
- Do you have chest pain, shortness of breath, heart disease, or have you had any surgery on your heart arteries?
- •
- Do you have risk factors for heart disease (such as high blood pressure, high cholesterol, obesity, diabetes, smoking, strong family history of heart disease, or you are postmenopausal or a male over 40)?
- •
- Do you have any problems with blood circulation in your arms or legs, fingers, or toes?
- •
- Are you pregnant? Do you think you might be pregnant? Are you trying to become pregnant? Are you sexually active and not using birth control?
- •
- D.H.E. 45 may cause preterm labor. D.H.E. 45 should be avoided during pregnancy. Talk to your healthcare provider right away if you are pregnant or want to become pregnant.
- •
- Are you breastfeeding?
- •
- D.H.E. 45 may reduce breast milk supply and pass into your breast milk. D.H.E. 45 may be harmful to your baby. Do not breastfeed your baby while taking D.H.E. 45 and for 3 days after you use D.H.E. 45. Talk with your healthcare provider about the best way to feed your baby if you take D.H.E. 45.
- •
- Have you ever had to stop taking this or any other medication because of an allergy or bad reaction?
- •
- Are you taking any other migraine medications, erythromycin or other antibiotics, or medications for blood pressure prescribed by your doctor, or other medicines obtained from your drugstore without a doctor's prescription?
- •
- Do you smoke?
- •
- Have you had, or do you have, any disease of the liver or kidney?
- •
- Is this headache different from your usual migraine attacks?
- •
- Are you using D.H.E. 45 (dihydroergotamine mesylate) Injection, USP or other dihydroergotamine mesylate containing drugs on a daily basis?
- •
- Are you taking a protease inhibitor for HIV therapy?
- •
- Are you taking a macrolide class of antibiotic?
Serious or potentially life-threatening reductions in blood flow to the brain or extremities have been reported rarely due to interactions between D.H.E. 45 (dihydroergotamine mesylate) Injection, USP and protease inhibitors or macrolide antibiotics.
REMEMBER TO TELL YOUR DOCTOR IF YOU HAVE ANSWERED “YES” TO ANY OF THESE QUESTIONS BEFORE YOU USE D.H.E. 45 (dihydroergotamine mesylate) Injection, USP
Side effects to watch out for
Although the following reactions rarely occur, they can be serious and should be reported to your physician immediately:
- •
- Numbness or tingling in your fingers and toes
- •
- Pain, tightness, or discomfort in your chest
- •
- Muscle pain or cramps in your arms and legs
- •
- Weakness in your legs
- •
- Temporary speeding or slowing of your heart rate
- •
- Swelling or itching
Dosage
Your doctor will have told you what dose to use for each migraine attack. Should you get another migraine attack in the same day as the attack you treated, you must not treat it with D.H.E. 45 (dihydroergotamine mesylate) Injection, USP unless at least 6 hours have elapsed since your last injection. No more than 6 mL of D.H.E. 45 (dihydroergotamine mesylate) Injection, USP should be injected during a 1-week period. D.H.E. 45 (dihydroergotamine mesylate) Injection, USP is not intended to be used on a prolonged daily basis.
Learn what to do in case of an Overdose
If you have used more medication than you have been instructed, contact your doctor, hospital emergency department, or nearest poison control center immediately.
How to use the D.H.E. 45 (dihydroergotamine mesylate) Injection, USP
- 1.
- Use Available Training Materials
- 1.
- Read and follow the instructions in the patient instruction booklet which is provided with the D.H.E. 45 (dihydroergotamine mesylate) Injection, USP package before attempting to use the product.
- 2.
- If there are any questions concerning the use of your D.H.E. 45 (dihydroergotamine mesylate) Injection, USP, ask your doctor or pharmacist.
- 2.
- Preparing for the Injection
- 3.
- Carefully examine the ampul (glass vial) of D.H.E. 45 (dihydroergotamine mesylate) Injection, USP for any cracks or breaks, and the liquid for discoloration, cloudiness, or particles. If any of these defects are present, use a new ampul, make certain it is intact, and return the defective ampul to your doctor or pharmacy. Once you open an ampul, if it is not used within an hour, it should be thrown away.
- 3.
- Locating an Injection Site
- 4.
- Administer your subcutaneous injection in the middle of your thigh, well above the knee.
- 4.
- Drawing the Medication into the Syringe
- 5.
- Wash your hands thoroughly with soap and water.
- 6.
- Check the dose of your medication.
- 7.
- Look to see if there is any liquid at the top of the ampul. If there is, gently flick the ampul with your finger to get all the liquid into the bottom portion of the ampul.
- 8.
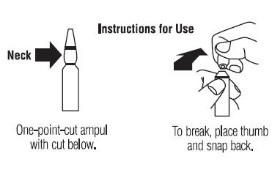
- Hold the bottom of the ampul in one hand. Clean the ampul neck with an alcohol wipe using the other hand. To break, place the alcohol wipe around the neck of the ampul and break it open by pressing your thumb against the neck of the ampul.
- 9.
- Tilt the ampul down at a 45° angle. Insert the needle into the solution in the ampul.
- 10.
- Draw up the medication by pulling back the plunger slowly and steadily until you reach your dose.
- 11.
- Check the syringe for air bubbles. Hold it with the needle pointing upward. If there are air bubbles, tap your finger against the barrel of the syringe to get the bubbles to the top. Slowly and carefully push the plunger up so that the bubbles are pushed out through the needle and you see a drop of medication.
- 12.
- When there are no air bubbles, check the dose of the medication. If the dose is incorrect, repeat steps e, f, g, and h until you draw up the right dose.
- 13.
- Preparing the Injection Site
- 5.
- With a new alcohol wipe, clean the selected injection site thoroughly with a firm, circular motion from inside to outside. Wait for the injection site to dry before injecting.
- 14.
- Administering the Injection
- 6.
- Hold the syringe/needle in your right hand.
- 7.
- With your left hand, firmly grasp about a 1-inch fold of skin at the injection site.
- 8.
- Push the needle shaft, bevel side up, all the way into the fold of skin at a 45° to 90° angle, then release the fold of skin.
- 9.
- While holding the syringe with your left hand, use your right hand to draw back slightly on the plunger.
- 10.
- If you do not see any blood coming back into the syringe, inject the medication by pushing down on the plunger. If you do see blood in the syringe, that means the needle has penetrated a vein. If this happens, pull the needle/syringe out of the skin slightly and draw back on the plunger again. If no blood is seen this time, inject the medication.
- 11.
- Use your right hand to pull the needle out of your skin quickly at the same angle you injected it. Immediately press the alcohol wipe on the injection site and rub.
Check the expiration date printed on the ampul containing medication. If the expiration date has passed, do not use it.
Answers to Patients' Questions About D.H.E. 45 (dihydroergotamine mesylate) Injection, USP
What if I need help in using my D.H.E. 45 (dihydroergotamine mesylate) Injection, USP?
If you have any questions or if you need help in opening, putting together, or using D.H.E. 45 (dihydroergotamine mesylate) Injection, USP, speak to your doctor or pharmacist.
How much medication should I use and how often?
Your doctor will have told you what dose to use for each migraine attack. Should you get another migraine attack in the same day as the attack you treated, you must not treat it with D.H.E. 45 (dihydroergotamine mesylate) Injection, USP unless at least 6 hours have elapsed since your last injection. No more than 6 mL of D.H.E. 45 (dihydroergotamine mesylate) Injection, USP should be injected during a 1-week period. Do not use more than this amount unless instructed to do so by your doctor. D.H.E. 45 (dihydroergotamine mesylate) Injection, USP is not intended for chronic daily use.
If you have any other unanswered questions about D.H.E. 45 (dihydroergotamine mesylate) Injection, USP, consult your doctor or pharmacist.
*Trademark of PDR Network, LLC
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
Jubilant HollisterStier General Partnership
16751 Trans-Canada Highway
Kirkland, Quebec H9H 4J4, Canada
®/™ are trademarks of Bausch Health Companies Inc. or its affiliates.
© 2022 Bausch Health Companies Inc. or its affiliates
04/2022
9614602
| D.H.E. 45
dihydroergotamine mesylate injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bausch Health US, LLC (831922468) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Jubilant HollisterStier General Partnership | 246762764 | MANUFACTURE(66490-041) | |