CHILDRENS PAIN RELIEVER GRAPE FLAVOR- acetaminophen tablet, chewable
Valu Merchandisers Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Best Choice 44-452-Delisted
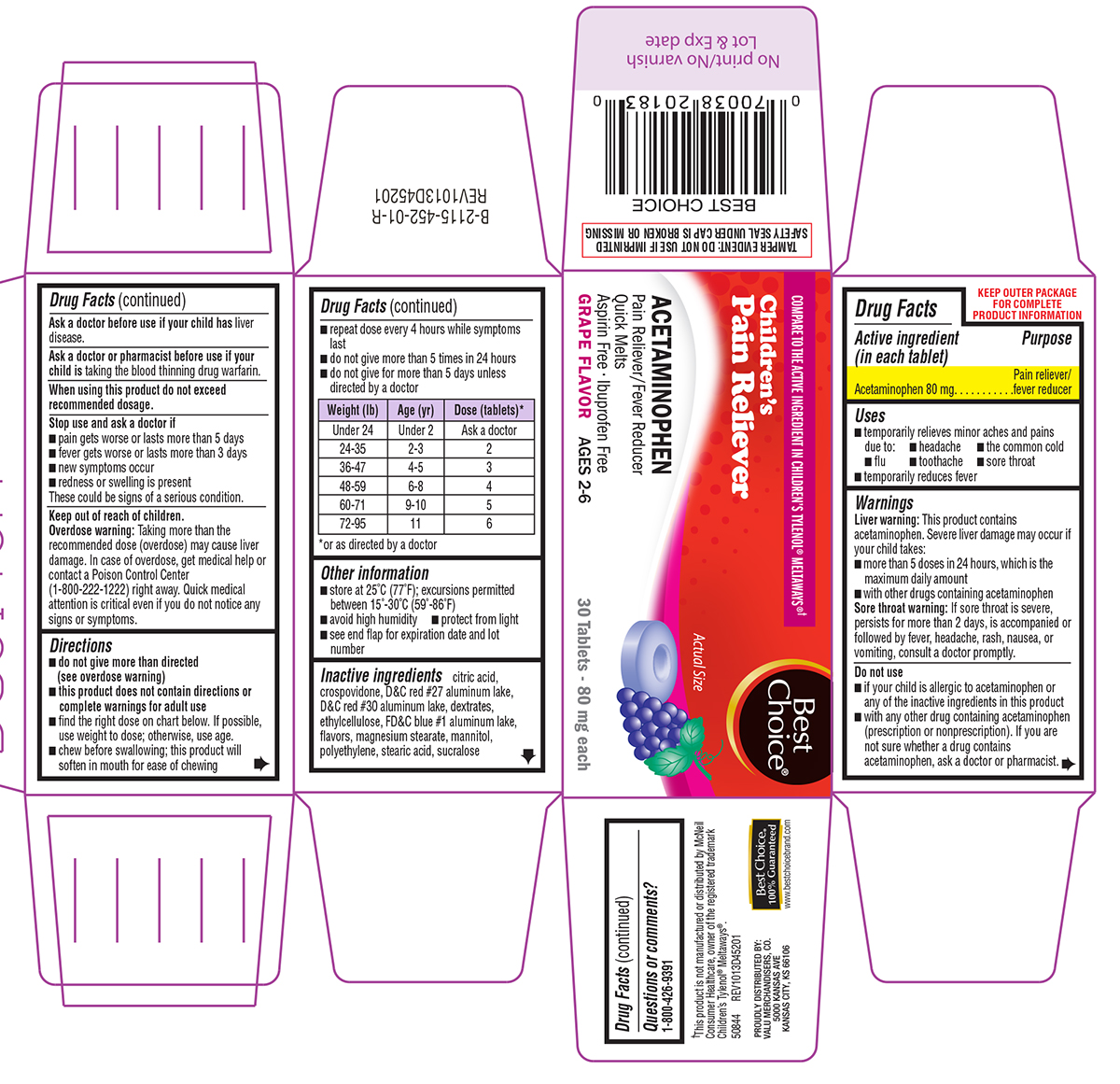
Uses
- temporarily relieves minor aches and pains due to:
- headache
- the common cold
- flu
- toothache
- sore throat
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes:
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Stop use and ask a doctor if
- pain gets worse or lasts more than 5 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical even if you do not notice any signs or symptoms.
Directions
-
do not give more than directed
(see overdose warning) - this product does not contain directions or complete warnings for adult use
- find the right dose on chart below. If possible, use weight to dose; otherwise, use age.
- chew before swallowing; this product will soften in mouth for ease of chewing
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- do not give for more than 5 days unless directed by a doctor
| Weight (lb) | Age (yr) | Dose (tablets)* |
| Under 24 | Under 2 | Ask a doctor |
| 24-35 | 2-3 | 2 |
| 36-47 | 4-5 | 3 |
| 48-59 | 6-8 | 4 |
| 60-71 | 9-10 | 5 |
| 72-95 | 11 |
6 |
*or as directed by a doctor
Other information
- store at 25ºC (77ºF); excursions permitted between 15°-30°C (59°-86°F)
- avoid high humidity
- protect from light
- see end flap for expiration date and lot number
Inactive ingredients
citric acid, crospovidone, D&C red #27 aluminum lake, D&C red #30 aluminum lake, dextrates, ethylcellulose, FD&C blue #1 aluminum lake, flavors, magnesium stearate, mannitol, polyethylene, stearic acid, sucralose
Principal Display Panel
COMPARE TO THE ACTIVE INGREDIENT IN CHILDREN'S TYLENOL® MELTAWAYS®†
Best Choice®
Children's
Pain Reliever
ACETAMINOPHEN
Pain Reliever/Fever Reducer
Quick Melts
Aspirin Free • Ibuprofen Free
GRAPE FLAVOR AGES 2-6
Actual Size
30 Tablets - 80 mg each
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
†This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Children's Tylenol® Meltaways®.
50844 REV1013D45201
PROUDLY DISTRIBUTED BY:
VALU MERCHANDISERS, CO.
5000 KANSAS AVE
KANSAS CITY, KS 66106
Best Choice®
100% Guaranteed
www.bestchoicebrand.com
Best Choice 44-452
| CHILDRENS PAIN RELIEVER
GRAPE FLAVOR
acetaminophen tablet, chewable |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Valu Merchandisers Company (868703513) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(63941-452) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | pack(63941-452) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(63941-452) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | pack(63941-452) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(63941-452) | |