Label: ACYCLOVIR ointment
- NDC Code(s): 70771-1371-1, 70771-1371-2
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated November 5, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
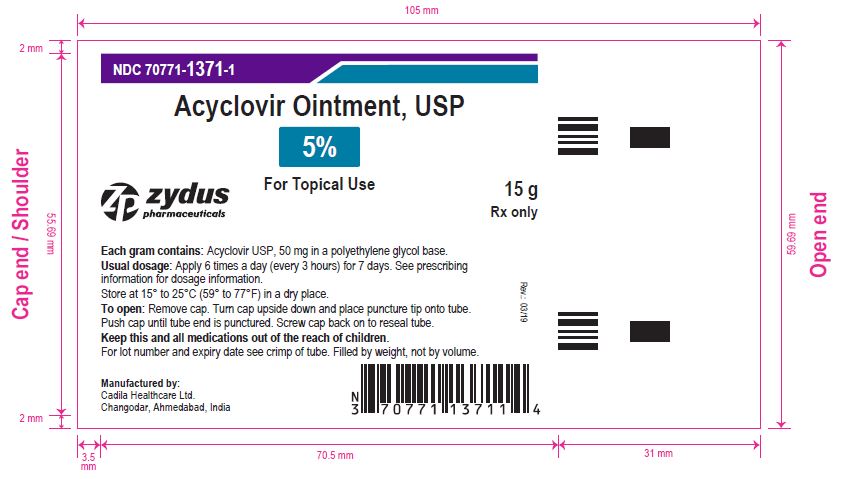
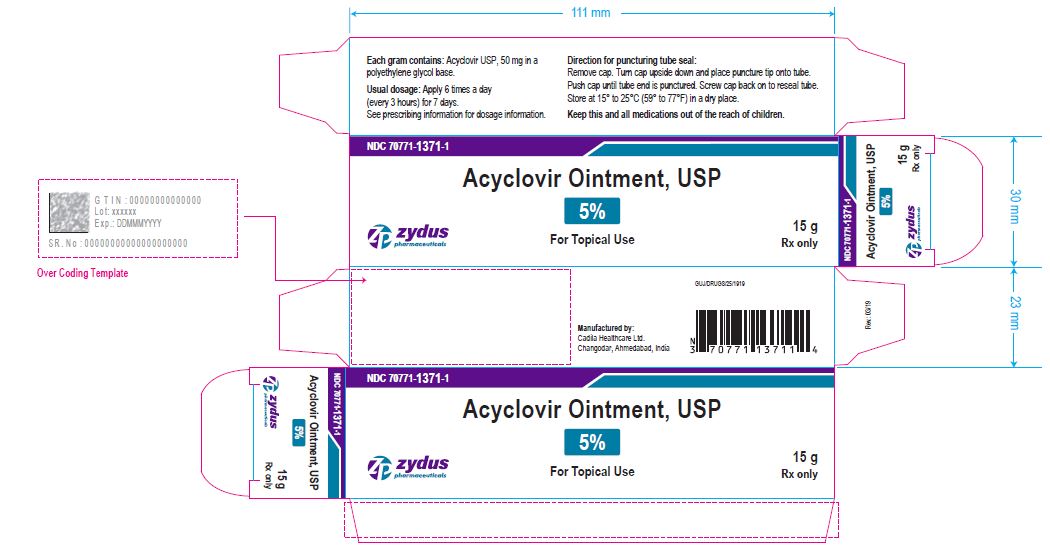
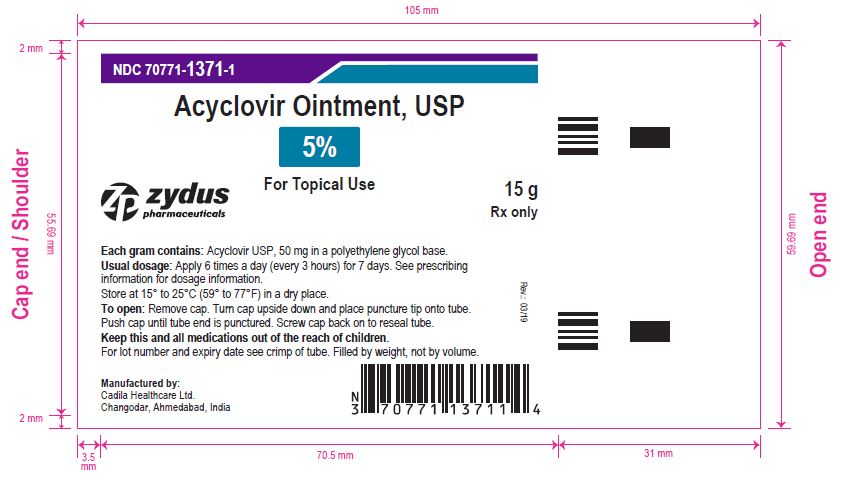
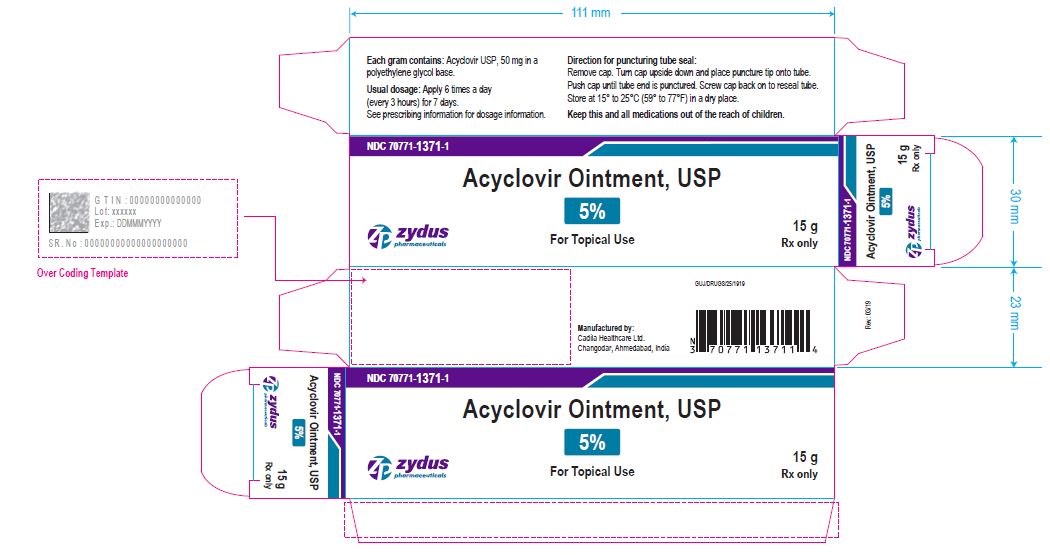
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACYCLOVIR
acyclovir ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1371 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACYCLOVIR (UNII: X4HES1O11F) (ACYCLOVIR - UNII:X4HES1O11F) ACYCLOVIR 50 mg in 1 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color WHITE (OPAQUE WHITE TO OFF-WHITE) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1371-1 1 in 1 CARTON 03/21/2019 1 15 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:70771-1371-2 1 in 1 CARTON 03/21/2019 2 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205974 03/21/2019 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 650650802 ANALYSIS(70771-1371) , MANUFACTURE(70771-1371)