BOVATEC 20- lasalocid sodium liquid
Alpharma, LLC.
----------
Bovatec®
Liquid 20
CATTLE: For improved feed efficiency and increased rate of weight gain when used in medicated feeds for cattle fed in confinement for slaughter. For increased rate of weight gain when used in medicated feeds for pasture cattle (slaughter, stocker, feeder cattle, and dairy and beef replacement heifers).
For control of coccidiosis caused by Eimeria bovis and E. zuernii in cattle up to 800 lbs.
SHEEP: For prevention of coccidiosis caused by Eimeria ovina, E. crandallis, E. ovinoidalis (E. ninakohlyakimovae), E. parva and E. intricata in sheep maintained in confinement.
Each pound contains 90.7 grams (20%) of lasalocid (as lasalocid sodium activity) in a carrier suitable for incorporation in liquid feed supplements.
IMPORTANT: Handling Information - Must be thoroughly mixed in feeds before use. When mixing and handling lasalocid liquid premix, use protective clothing and impervious gloves. Avoid contact with eyes. Operators should wash hands thoroughly with soap and water after handling.
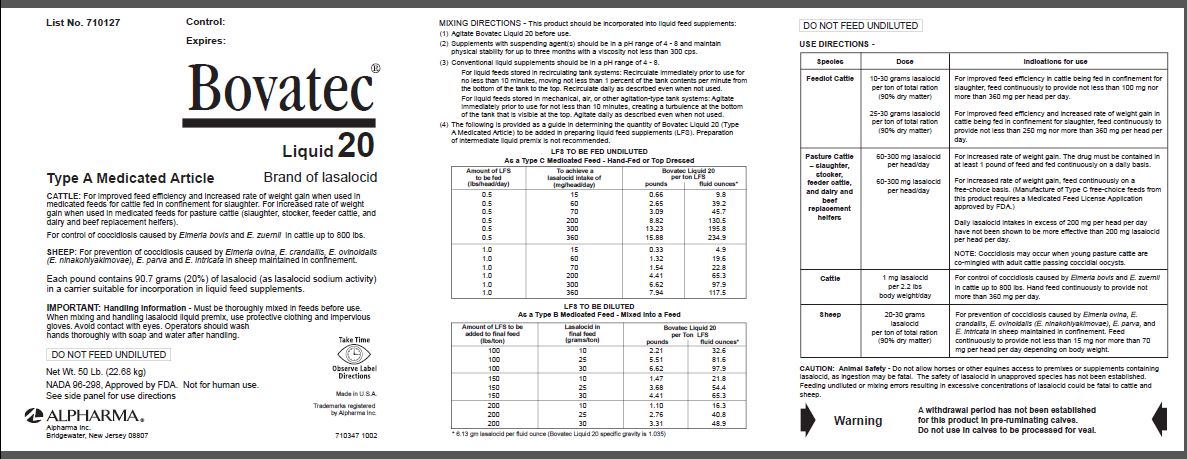
MIXING DIRECTIONS
This product should be incorporated into liquid feed supplements:
(1) Agitate Bovatec Liquid 20 before use.
(2) Supplements with suspending agent(s) should be in a pH range of 4 - 8 and maintain physical stability for up to three months with a viscosity not less than 300 cps.
(3) Conventional liquid supplements should be in a pH range of 4 - 8.
For liquid feeds stored in recirculating tank systems: Recirculate immediately prior to use for no less than 10 minutes, moving not less than 1 percent of the tank contents per minute from the bottom of the tank to the top. Recirculate daily as described even when not used.
For liquid feeds stored in mechanical, air, or other agitation-type tank systems: Agitate immediately prior to use for not less than 10 minutes, creating a turbulence at the bottom of the tank that is visible at the top. Agitate daily as described even when not used.
(4) The following is provided as a guide in determining the quantity of Bovatec Liquid 20 (Type A Medicated Article) to be added in preparing liquid feed supplements (LFS). Preparation of intermediate liquid premix is not recommended.
|
|||
| LFS TO BE FED UNDILUTED As a Type C Medicated Feed - Hand-Fed or Top Dressed |
|||
| Amount of LFS to be fed (lbs/head/day) | To achieve a lasalocid intake of (mg/head/day) | Bovatec Liquid 20 per ton LFS | |
| pounds | fluid ounces* | ||
| 0.5 | 15 | 0.66 | 9.8 |
| 0.5 | 60 | 2.65 | 39.2 |
| 0.5 | 70 | 3.09 | 45.7 |
| 0.5 | 200 | 8.82 | 130.5 |
| 0.5 | 300 | 13.23 | 195.8 |
| 0.5 | 360 | 15.88 | 234.9 |
| 1.0 | 15 | 0.33 | 4.9 |
| 1.0 | 60 | 1.32 | 19.6 |
| 1.0 | 70 | 1.54 | 22.8 |
| 1.0 | 200 | 4.41 | 65.3 |
| 1.0 | 300 | 6.62 | 97.9 |
| 1.0 | 360 | 7.94 | 117.5 |
| LFS TO BE DILUTED As a Type B Medicated Feed - Mixed into a Feed |
|||
| Amount of LFS to be added to final feed (lbs/ton) | Lasalocid in final feed (grams/ton) | Bovatec Liquid 20 per Ton LFS | |
| pounds | fluid ounces* | ||
| 100 | 10 | 2.21 | 32.6 |
| 100 | 25 | 5.51 | 81.6 |
| 100 | 30 | 6.62 | 97.9 |
| 150 | 10 | 1.47 | 21.8 |
| 150 | 25 | 3.68 | 54.4 |
| 150 | 30 | 4.41 | 65.3 |
| 200 | 10 | 1.10 | 16.3 |
| 200 | 25 | 2.76 | 40.8 |
| 200 | 30 | 3.31 | 48.9 |
DO NOT FEED UNDILUTED
USE DIRECTIONS
| Species | Dose | Indications for use |
|---|---|---|
| Feedlot Cattle | 10-30 grams lasalocid per ton of total ration (90% dry matter) | For improved feed efficiency in cattle being fed in confinement for slaughter, feed continuously to provide not less than 100 mg nor more than 360 mg per head per day. |
| 25-30 grams lasalocid per ton of total ration (90% dry matter) | For improved feed efficiency and increased rate of weight gain in cattle being fed in confinement for slaughter, feed continuously to provide not less than 250 mg nor more than 360 mg per head per day. | |
| Pasture Cattle – slaughter, stocker, feeder cattle, and dairy and beef replacement heifers | 60-300 mg lasalocid per head/day | For increased rate of weight gain. The drug must be contained in at least 1 pound of feed and fed continuously on a daily basis. |
| 60-300 mg lasalocid per head/day | For increased rate of weight gain, feed continuously on a free-choice basis. (Manufacture of Type C free-choice feeds from this product requires a Medicated Feed License Application approved by FDA.) | |
| Daily lasalocid intakes in excess of 200 mg per head per day have not been shown to be more effective than 200 mg lasalocid per head per day. | ||
| NOTE: Coccidiosis may occur when young pasture cattle are comingled with adult cattle passing coccidial oocysts. | ||
| Cattle | 1 mg lasalocid per 2.2 lbs body weight/day | For control of coccidiosis caused by Eimeria bovis and E. zuernii in cattle up to 800 lbs. Hand feed continuously to provide not more than 360 mg per day. |
| Sheep | 20-30 grams lasalocid per ton of total ration (90% dry matter) | For prevention of coccidiosis caused by Eimeria ovina, E. crandallis, E. ovinoidalis (E. ninakohlyakimovae), E. parva, and E. intricata in sheep maintained in confinement. Feed continuously to provide not less than 15 mg nor more than 70 mg per head per day depending on body weight. |
CAUTION: Animal Safety - Do not allow horses or other equines access to premixes or supplements containing lasalocid, as ingestion may be fatal. The safety of lasalocid in unapproved species has not been established. Feeding undiluted or mixing errors resulting in excessive concentrations of lasalocid could be fatal to cattle and sheep.
Warning
A withdrawal period has not been established for this product in pre-ruminating calves.
Do not use in calves to be processed for veal.
DO NOT FEED UNDILUTED
Net Wt. 50 Lb. (22.68 kg)
NADA 96-298, Approved by FDA. Not for human use.
See side panel for use directions

Made in U.S.A.
Trademarks registered
by Alpharma Inc.
710347 1002
List No. 710127
Alpharma Inc.
Bridgewater, New Jersey 08807
PRINCIPAL DISPLAY PANEL - 50 lb Drum
List No. 710127
Control
Expires:
Bovatec®
Liquid 20
Type A Medicated Article
Brand of lasalocid
CATTLE: For improved feed efficiency and increased rate of weight gain when used in
medicated feeds for cattle fed in confinement for slaughter. For increased rate of weight
gain when used in medicated feeds for pasture cattle (slaughter, stocker, feeder cattle, and
dairy and beef replacement heifers).
For control of coccidiosis caused by Eimeria bovis and E. zuernii in cattle up to 800 lbs.
SHEEP: For prevention of coccidiosis caused by Eimeria ovina, E. crandallis, E. ovinoidalis
(E. ninakohlyakimovae), E. parva and E. intricata in sheep maintained in confinement.
Each pound contains 90.7 grams (20%) of lasalocid (as lasalocid sodium activity)
in a carrier suitable for incorporation in liquid feed supplements.
IMPORTANT: Handling Information - Must be thoroughly mixed in feeds before use.
When mixing and handling lasalocid liquid premix, use protective clothing and impervious
gloves. Avoid contact with eyes. Operators should wash
hands thoroughly with soap and water after handling.
DO NOT FEED UNDILUTED
Net Wt. 50 Lb. (22.68 kg)
NADA 96-298, Approved by FDA. Not for human use.
See side panel for use directions
Alpharma®
Alpharma Inc.
Bridgewater, New Jersey 08807
Take Time
Observe Label
Directions
Made in U.S.A.
Trademarks registered
by Alpharma Inc.
710347 1002
| BOVATEC 20
lasalocid sodium liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Alpharma, LLC. (070954094) |