Label: IRON HYDROGENATED DEXTRAN- iron injection
- NDC Code(s): 13985-520-02, 13985-520-05
- Packager: MWI Veterinary Supply, Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated October 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
HEMATINIC
For Prevention and Treatment of Iron Deficiency Anemia in Baby Pigs
NOT FOR HUMAN USE
KEEP OUT OF REACH OF CHILDREN
Approved by FDA under NADA # 138-255
DESCRIPTION
IRON HYDROGENATED DEXTRAN INJECTION is a sterile solution containing 100 mg of elemental iron per mL, stabilized with a low molecular weight hydrogenated dextran and 0.5% phenol as a preservative.
-
INDICATIONS
For the prevention and treatment of anemia due to iron deficiency in baby pigs.
Iron deficiency anemia occurs commonly in the suckling pig, often within the first few days following birth. As body size and blood volume increase rapidly from the first few days following birth, hemoglobin levels in the blood fall due to diminishing iron reserves which cannot be replaced adequately from iron in the sow's milk. Thus, the need for a readily available outside source of iron is apparent. Without this additional iron many newborn pigs may develop anemia within the first few days of life. Anemia retards growth and lowers the body defenses against disease; scours, pneumonia, and other conditions may develop. Death from anemia or infections may occur, sometimes destroying entire litters.
-
DOSAGE
For intramuscular injection only.
For Prevention of iron deficiency anemia, administer 1 mL (100 mg iron) at 2 to 4 days of age.
For Treatment of iron deficiency anemia, administer 1 mL (100 mg iron). Treatment may be repeated in 10 days.
DIRECTIONS FOR USE
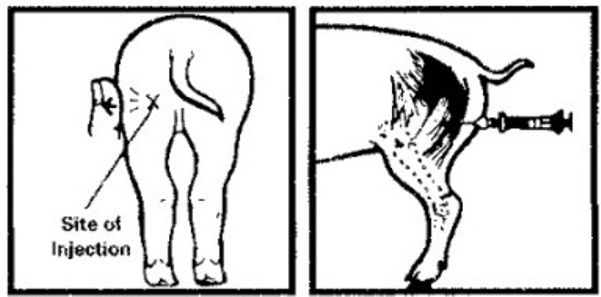
Disinfect rubber stopper of vial and injection site with a cotton ball moistened with rubbing alcohol or other suitable disinfectant. Use a small needle (20 gauge, 5/8 inch) and syringe that have been sterilized by boiling in water for 20 minutes. Injection should be made intramuscularly into the back of the ham (see diagram below). Apply tension to the skin at the injection site by pulling skin and fat downward with pressure from the thumb or fingers. Inject into the muscle to a depth of approximately 1/2 inch. Use a clean, sterile needle and syringe for each pig to avoid spreading infection.
- PRECAUTIONS
- SIDE EFFECTS
- HOW SUPPLIED
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IRON HYDROGENATED DEXTRAN
iron injectionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:13985-520 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 100 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-520-02 100 mL in 1 VIAL 2 NDC:13985-520-05 250 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA138255 11/19/2011 Labeler - MWI Veterinary Supply, Inc. (019926120)




