POTASSIUM CHLORIDE- potassium chloride injection, solution, concentrate
B. Braun Medical Inc.
----------
Potassium Chloride for Injection Concentrate USP
(2 mEq K+/mL)
PHARMACY BULK PACKAGE
NOT FOR DIRECT INFUSION
FOR INTRAVENOUS INFUSION ONLY
MUST BE DILUTED PRIOR TO INJECTION
DESCRIPTION
A pharmacy bulk package is a container of a sterile preparation for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture service and are restricted to the preparation of admixtures for intravenous infusion.
Potassium Chloride for Injection Concentrate USP is a sterile, nonpyrogenic, concentrated solution of Potassium Chloride USP in Water for Injection USP to be administered by intravenous infusion only after dilution in a larger volume of fluid. No bacteriostatic or antimicrobial agent has been added.
Each 100 mL of Potassium Chloride contains:
Potassium Chloride USP 14.9 g; Water for Injection USP qs
pH: 5.4 (4.0–8.0); Calculated Osmolarity: 4000 mOsmol/liter
Concentration of Electrolytes (mEq/mL): Potassium 2; Chloride 2
The formula of the active ingredient is:
| Ingredient | Molecular Formula | Molecular Weight |
|---|---|---|
| Potassium Chloride USP | KCl | 74.55 |
Potassium chloride injection (approximately diluted) is a parenteral fluid and electrolyte replenisher.
CLINICAL PHARMACOLOGY
Potassium is the chief cation of body cells (160 mEq/liter of intracellular water) and is concerned with the maintenance of body fluid composition and electrolyte balance. Potassium participates in carbohydrate utilization and protein synthesis, and is critical in the regulation of nerve conduction and muscle contraction, particularly in the heart. Chloride, the major extracellular anion, closely follows the metabolism of sodium and changes in the acid-base balance of the body are reflected by changes in the chloride concentration.
Normally about 80 to 90% of the potassium intake is excreted in the urine, the remainder in the stools and to a small extent, in the perspiration. The kidney does not conserve potassium well so that during fasting, or in patients on a potassium-free diet, potassium loss from the body continues resulting in potassium depletion. A deficiency of either potassium or chloride will lead to a deficit of the other.
INDICATIONS AND USAGE
Potassium Chloride for Injection Concentrate USP is indicated in the treatment of potassium deficiency states when oral replacement is not feasible.
This is a concentrated solution which is intended for use in a pharmacy admixture service and is restricted to the preparation of admixtures for intravenous infusion.
CONTRAINDICATIONS
Potassium Chloride for Injection Concentrate USP is contraindicated in diseases where high potassium levels may be encountered, in patients with hyperkalemia, renal failure and in conditions in which potassium retention is present, or where additives of potassium and chloride could be clinically detrimental.
WARNINGS
Strongly Hypertonic Solution. Must be properly diluted and thoroughly mixed before injection.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
This concentrated solution of potassium chloride is for use in intravenous admixtures only and must not be used undiluted for direct patient injection. Direct patient injection of potassium chloride at this concentration may be instantaneously fatal.
The administration of intravenous solutions can cause fluid and/or solute overload resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema. The risk of dilutional states is inversely proportional to the electrolyte concentration. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentration.
To avoid potassium intoxication, do not infuse these solutions rapidly. In patients with renal insufficiency, administration of potassium chloride may cause potassium intoxication and life-threatening hyperkalemia.
In patients with diminished renal function, administration of solutions containing potassium ions may result in potassium retention.
PRECAUTIONS
General
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation. Significant deviations from normal concentrations may require the use of additional electrolyte supplements, or the use of electrolyte-free dextrose solutions to which individualized electrolyte supplements may be added.
Potassium therapy should be guided primarily by serial electrocardiograms, especially in patients receiving digitalis. Serum potassium levels are not necessarily indicative of tissue potassium levels. Solutions containing potassium should be used with caution in the presence of cardiac disease, particularly in the presence of renal disease, and in such instances, cardiac monitoring is recommended.
Solutions containing potassium in a greater concentration than 40 mEq/liter may result in significant irritation to peripheral or central veins.
For peripheral administration of solutions containing potassium, slowly infuse the solution through a small bore needle, placed well within the lumen of a large vein. Carefully avoid infiltration.
Solutions containing dextrose should be used with caution in patients with overt or known subclinical diabetes mellitus, or carbohydrate intolerance for any reason.
If the administration is controlled by a pumping device, care must be taken to discontinue pumping action before the container runs dry or air embolism may result.
To minimize the risk of possible incompatibilities arising from mixing this solution with other additives that may be prescribed, the final infusate should be inspected for cloudiness or precipitation immediately after mixing, prior to administration, and periodically during administration.
Use only if solution is clear and vacuum is present. Discard the container no later than 4 hours after initial closure puncture.
Pregnancy
(I) Teratogenic effects. Pregnancy Category C
Animal reproduction studies have not been conducted with potassium chloride injection. It is also not known whether potassium chloride injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Potassium chloride injection should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS
Reactions which may occur because of the potassium-containing solutions or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection or extravasation, hypervolemia, and hyperkalemia.
To rapid infusion of hypertonic solutions may cause local pain and, rarely, vein irritation. Rate of administration should be adjusted according to tolerance.
Reactions reported with the use of potassium-containing solutions include nausea, vomiting, abdominal pain and diarrhea. The signs and symptoms of potassium intoxication include paresthesias of the extremities, areflexia, muscular or respiratory paralysis, mental confusion, weakness, hypotension, cardiac arrhythmias, heart block, electrocardiographic abnormalities and cardiac arrest. Potassium deficits result in disruption of neuromuscular function, and intestinal ileus and dilatation.
If infused in large amounts, chloride ions may cause a loss of bicarbonate ions, resulting in an acidifying effect.
Potassium-containing solutions are intrinsically irritating to tissues. Therefore, extreme care should be taken to avoid perivascular infiltration. Local tissue necrosis and subsequent sloughing may result if extravasation occurs. Chemical phlebitis and venospasm have also been reported.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
OVERDOSAGE
In the event of fluid overload during parenteral therapy, reevaluate the patient's condition and institute appropriate corrective treatment.
In the event of overdosage with potassium-containing solutions, discontinue the infusion immediately and institute corrective therapy to reduce serum potassium levels.
Treatment of hyperkalemia includes the following:
- Dextrose Injection USP, 10% or 25%, containing 10 units of crystalline insulin per 20 grams of dextrose administered intravenously, 300 to 500 mL per hour.
- Absorption and exchange of potassium using sodium or ammonium cycle cation exchange resin, orally and as retention enema.
- Hemodialysis and peritoneal dialysis. The use of potassium-containing foods or medications must be eliminated. However, in cases of digitalization, too rapid a lowering of plasma potassium concentration can cause digitalis toxicity.
DOSAGE AND ADMINISTRATION
Not for direct patient injection. Potassium Chloride for Injection Concentrate USP, Pharmacy Bulk Package is for preparation of intravenous admixtures only and must be diluted before administration. Care must be taken to ensure there is complete mixing of the potassium chloride with the large volume fluid, particularly if soft or bag type containers are used.
Dosage and rate of administration are to be directed by the physician and are dependent upon the specific conditions of each patient (e.g., age, weight, clinical condition of the patient and laboratory determinations). Frequent laboratory determinations and clinical evaluation are essential to monitor changes in electrolyte concentrations, and fluid and electrolyte balance during prolonged parenteral therapy.
If the serum potassium level is greater than 2.5 mEq/liter, potassium can be given at a rate not to exceed 10 mEq/hour and in a concentration of up to 40 mEq/liter. The 24 hour total dose should not exceed 200 mEq.
If urgent treatment is indicated (serum potassium level less than 2.0 mEq/liter and electrocardiographic changes and/or muscle paralysis), potassium chloride may be infused very cautiously at a rate of up to 40 mEq/hour. In such cases, continuous cardiac monitoring is essential. As much as 400 mEq may be administered in a 24 hour period. In critical conditions, potassium chloride may be administered in saline (unless contraindicated) rather than in dextrose containing fluids, as dextrose may lower serum potassium levels.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Directions for Use of Pharmacy Bulk Package in B. Braun Glass Containers with Solid Stoppers
Warning: Not for direct infusion. For preparation of admixtures for intravenous infusion.
The pharmacy bulk package is for use in a Pharmacy Admixture Service only. Use of this product is restricted to a suitable work area, such as a laminar flow hood (or an equivalent clean air compounding area).
Additives should not be made to Pharmacy Bulk Packages.
Designed for use with a vented sterile dispensing set.
Before use, perform the following checks:
- Inspect each container. Read the label. Ensure solution is the one ordered and is within the expiration date. Verify that closure is black and is printed with the words "MUST BE DILUTED".
- Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter; check the bottle for cracks or other damage. In checking for cracks, do not be confused by normal surface marks and seams on bottom and sides of bottle. These are not flaws. Look for bright reflections that have depth and penetrate into the wall of the bottle. Reject any such bottle.
-
Remove plastic cap (See Figure 1).
-
Swab exposed stopper surface with a suitable disinfectant.
-
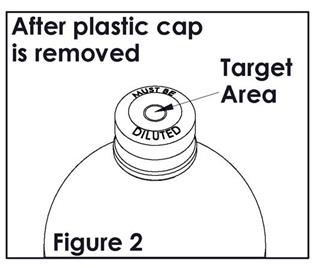
Check for vacuum at first puncture of the stopper. Insert the spike fully into the target area of the rubber stopper (See Figure 2) and promptly invert the bottle. Verify vacuum by observing rising air bubbles. Do not use the bottle if vacuum is not present. Refer to Directions for Use of set to be used.
-
If set insertion is not performed immediately following swabbing, swab stopper again with a suitable disinfectant.
The container closure may be penetrated only one time, utilizing a suitable sterile dispensing set which allows measured dispensing of the contents.
Transfer individual dose(s) to appropriate intravenous infusion solutions. Use of a syringe with needle is not recommended. Multiple entries increase the potential of the microbial and particulate contamination.
The withdrawal of container contents should be accomplished without delay using aseptic technique. Discard container no later than 4 hours after initial closure puncture.
The bottle may be stored under laminar flow hood at room temperature (25°C) after the closure has been entered. Date and time of container entry should be noted in the area designated on the container label.
HOW SUPPLIED
Potassium Chloride for Injection Concentrate USP (2 mEq K+/mL) is supplied sterile and nonpyrogenic in 250 mL glass containers with solid stoppers, Pharmacy Bulk Packages, packaged 12 per case.
| NDC | REF | Size |
|---|---|---|
| Potassium Chloride for Injection Concentrate USP | ||
| 0264-1940-20 | S9402-11 | 250 mL |
Rx only
Revised: April 2018
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
Y36-002-976 LD-426-3
PRINCIPAL DISPLAY PANEL - 250 mL Container Label
Potassium Chloride for
Injection Concentrate USP
(2 mEq K+/mL)
REF S9402-11
NDC 0264-1940-20
250 mL
PHARMACY BULK PACKAGE
NOT FOR DIRECT INFUSION
Each 100 mL contains:
Potassium Chloride USP 14.9 g; Water for Injection USP qs
pH: 5.4 (4.0-8.0); Calc. Osmolarity: 4000 mOsmol/liter
Electrolytes mEq/mL: Potassium 2; Chloride 2
Contains no more than 25 mcg/L of aluminum.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
Opened: Date Time
Sterile, nonpyrogenic. Pharmacy Bulk Package.
No antimicrobial or bacteriostatic agent has been added.
Recommended Storage: Room temperature (25°C).
Avoid excessive heat (40°C/104°F). Protect from freezing.
See Package Insert.
Usual Dosage and Directions for Use:
See Package Insert.
WARNING: Not for direct infusion.
For preparation of intravenous admixtures only.
Concentrated solution: dilute in suitable fluid prior
to administration.
Use only if solution is clear and vacuum is present.
A single entry through the vial closure should be made with
a sterile dispensing set. Transfer individual doses to
appropriate intravenous infusion solutions without delay.
Use of a syringe with needle is not recommended. The
above process should be carried out under a laminar
flow hood using aseptic technique.
Discard the container no later than 4 hours after initial
closure puncture.
Rx Only
Y37-002-548 LD-614-1
EXP
LOT

| POTASSIUM CHLORIDE
potassium chloride injection, solution, concentrate |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - B. Braun Medical Inc. (002397347) |