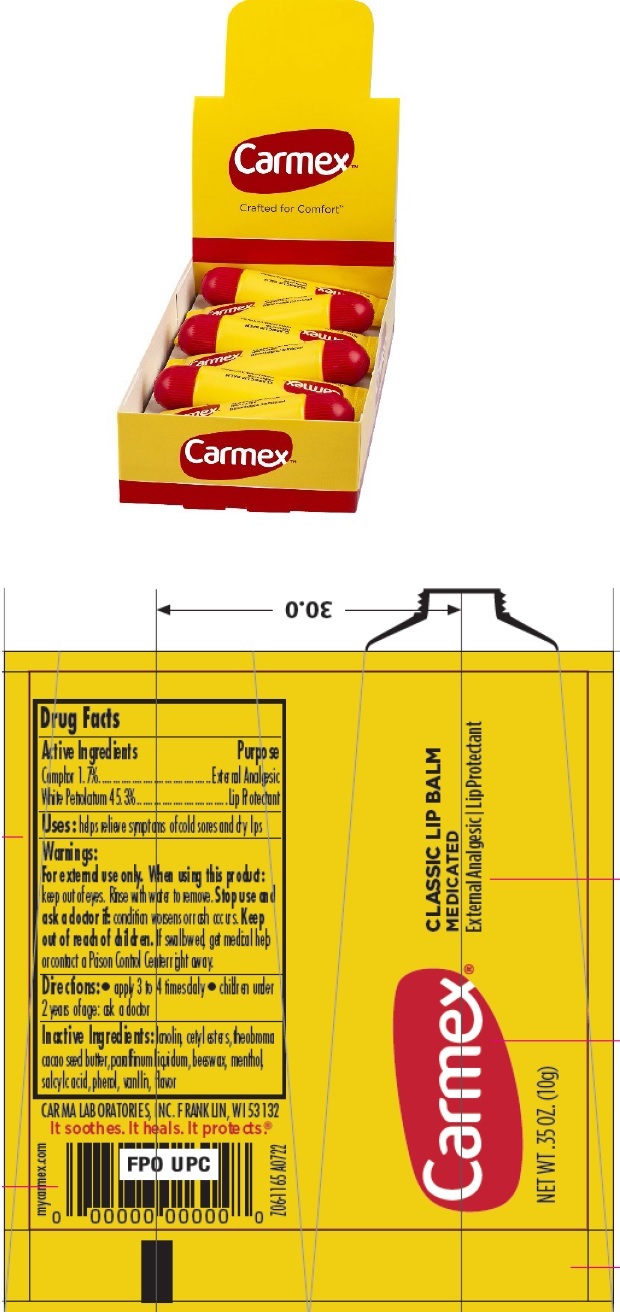
Label: CARMEX CLASSIC LIP BALM MEDICATED (camphor- synthetic, petrolatum salve
-
NDC Code(s):
10210-0022-1,
10210-0022-2,
10210-0022-3,
10210-0022-4, view more10210-0022-5, 10210-0022-6, 10210-0022-7
- Packager: Carma Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated August 21, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Package Labeling:
- Package Labeling:10210-0022-7
-
INGREDIENTS AND APPEARANCE
CARMEX CLASSIC LIP BALM MEDICATED
camphor (synthetic), petrolatum salveProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10210-0022 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1.7 g in 100 g WHITE PETROLATUM (UNII: B6E5W8RQJ4) (WHITE PETROLATUM - UNII:B6E5W8RQJ4) WHITE PETROLATUM 45.3 g in 100 g Inactive Ingredients Ingredient Name Strength LANOLIN (UNII: 7EV65EAW6H) CETYL ESTERS WAX (UNII: D072FFP9GU) COCOA BUTTER (UNII: 512OYT1CRR) PARAFFIN (UNII: I9O0E3H2ZE) YELLOW WAX (UNII: 2ZA36H0S2V) MENTHOL (UNII: L7T10EIP3A) SALICYLIC ACID (UNII: O414PZ4LPZ) PHENOL (UNII: 339NCG44TV) VANILLA (UNII: Q74T35078H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10210-0022-1 1 in 1 BLISTER PACK 09/26/2016 1 7.5 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:10210-0022-2 3 in 1 PACKAGE 09/26/2016 2 7.5 g in 1 JAR; Type 0: Not a Combination Product 3 NDC:10210-0022-3 7.5 g in 1 JAR; Type 0: Not a Combination Product 09/26/2016 4 NDC:10210-0022-4 1 in 1 BLISTER PACK 09/26/2016 4 10 g in 1 TUBE; Type 0: Not a Combination Product 5 NDC:10210-0022-5 3 in 1 PACKAGE 09/26/2016 5 10 g in 1 TUBE; Type 0: Not a Combination Product 6 NDC:10210-0022-6 10 g in 1 TUBE; Type 0: Not a Combination Product 09/26/2016 7 NDC:10210-0022-7 12 in 1 BOX 01/01/2023 7 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/26/2016 Labeler - Carma Laboratories, Inc. (006090153)