PROACTIV CLARIFYING NIGHT ACNE TREATMENT- salicylic acid cream
THE PROACTIV COMPANY LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
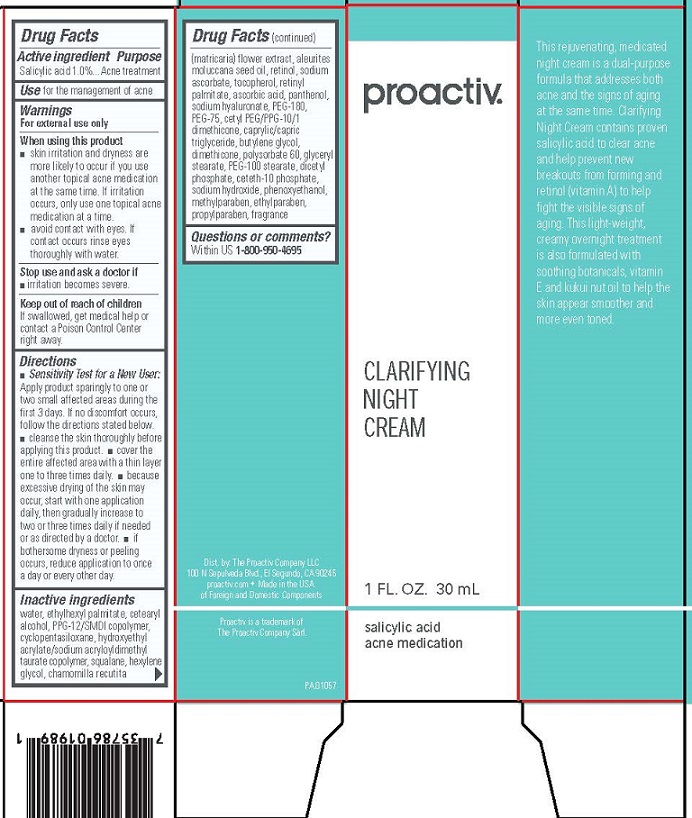
proactiv® SOLUTION Clarifying Night Cream
Salicylic Acid Acne Treatment
Drug Facts
Warnings
For external use only
When using this product
- using other topical acne medications at the same time or immediately following use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
- avoid unnecessary sun exposure and use a sunscreen.
Directions
- Cleanse the skin thoroughly before applying medication.
- Cover the entire affected area with a thin layer 1 to 3 times daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to 2 or 3 times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive Ingredients
water (aqua), ethylhexyl palmitate, cetearyl alcohol, PPG-12/SMDI copolymer, cyclopentasiloxane, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, squalane, chamomilla recutita (matricaria) flower extract, aleurites moluccana seed oil, retinol, sodium ascorbate, tocopherol, retinyl palmitate, ascorbic acid, panthenol, sodium hyaluronate, PEG-180, PEG-75, PEG-8 cetyl dimethicone, hexylene glycol, caprylic/capric triglyceride, propylene glycol, dimethicone, polysorbate 60, glyceryl stearate, PEG-100 stearate, dicetyl phosphate, ceteth-10 phosphate, sodium hydroxide, phenoxyethanol, methylparaben, ethylparaben, butylparaben, propylparaben, isobutylparaben, fragrance (parfum)
| PROACTIV
CLARIFYING NIGHT ACNE TREATMENT
salicylic acid cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - THE PROACTIV COMPANY LLC (080216357) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| VEE PAK, LLC | 874763303 | manufacture(11410-402) | |