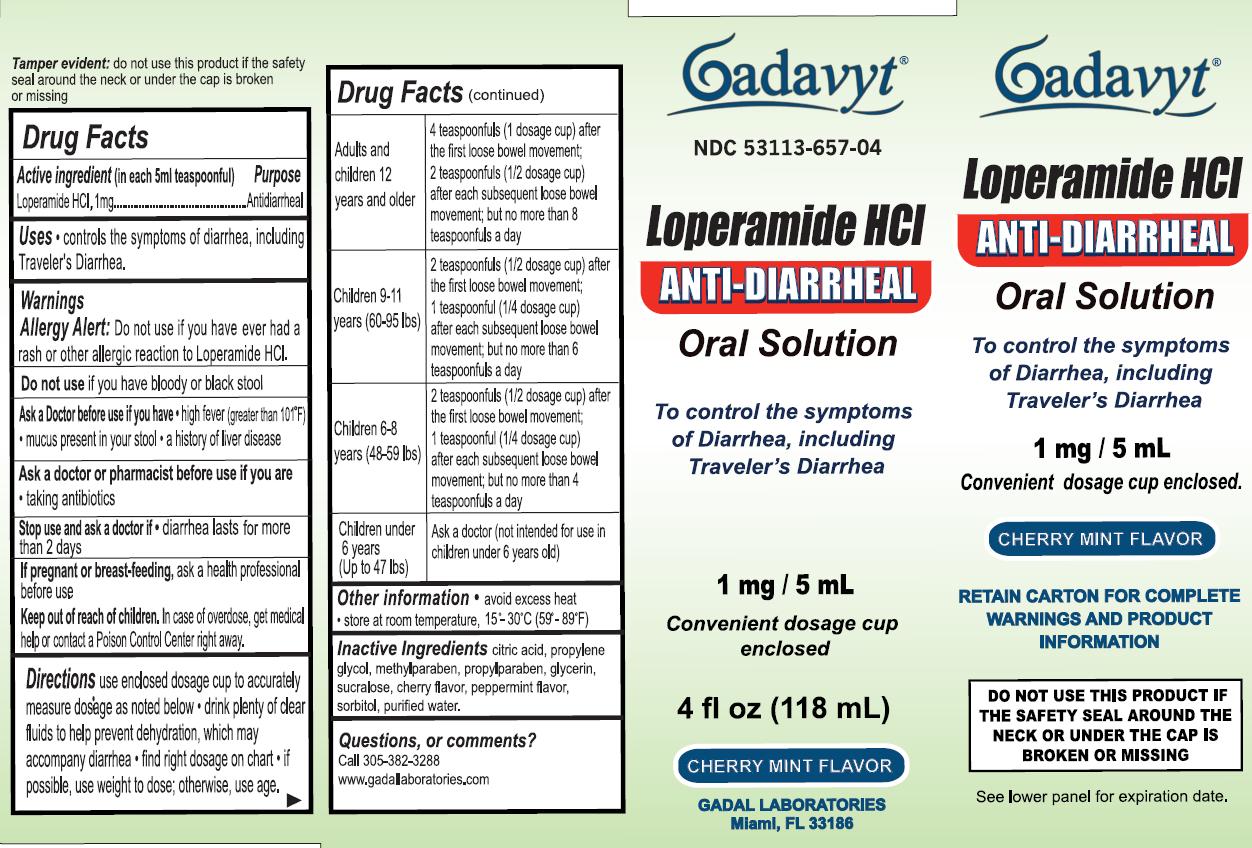
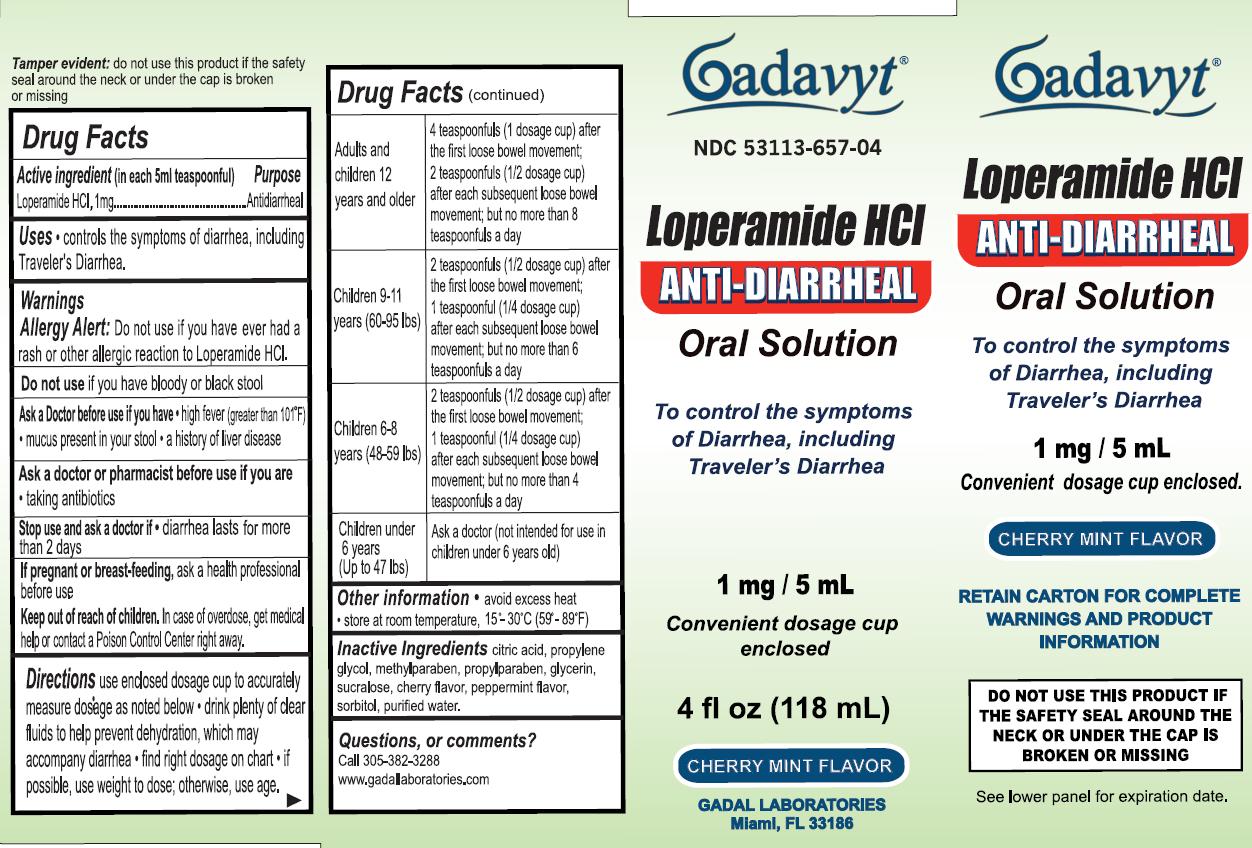
Label: GADAVYT LOPERAMIDE HCL- loperamide hydrochloride liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 53113-657-04 - Packager: Gadal Laboratories Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 22, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
DO NOT USE
Do not use if you have bloody or black stool
Ask a Doctor before use if you have
- high fiver (greater than 101 degrees F)
- mucus present in your stool
- a history of liver disease
Ask a doctor or pharmacist before use if you are taking antibiotics
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
-
DOSAGE & ADMINISTRATION
Directions
Use enclosed dosage cup to accurately measure dosage as noted below
- drink plenty of clear fluids to help prevent dehydration, which may accompany diarrhea
- find right dosage on chart
- if possible, use weight to dose; otherwise, use age.
Adults and children 12 years and older
4 teaspoonfuls (1 dosage cup) after the first loose bowel movement;
2 teaspoonfuls (1/2 dosage cup) after each subsequent loose bowel movement;
but no more than 8 teaspoonfuls a day
Children 9-11 (60-95 lbs)
2 teaspoonfuls (1/2 dosage cup) after the first loose bowel movement;
1 teaspoonful (1/4 dosage cup) after each subsequent loose bowel movement;
but no more than 6 teaspoonfuls a day
Children 6-8 (48-59 lbs)
2 teaspoonfuls (1/2 dosage cup) after the first loose bowel movement;
1 teaspoonfuls (1/4 dosage cup) after each subsequent loose bowel movement;
but no more than 4 teaspoonfuls a day
Children under 6 years (Up to 47 lbs)
Ask a doctor (not intended for use in children under 6 years old
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GADAVYT LOPERAMIDE HCL
loperamide hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53113-657 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOPERAMIDE HYDROCHLORIDE (UNII: 77TI35393C) (LOPERAMIDE - UNII:6X9OC3H4II) LOPERAMIDE HYDROCHLORIDE 1 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) GLYCERIN (UNII: PDC6A3C0OX) SUCRALOSE (UNII: 96K6UQ3ZD4) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor CHERRY (Cherry) , MINT (Mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53113-657-04 1 in 1 CARTON 1 118 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/01/2013 Labeler - Gadal Laboratories Inc (841305639)