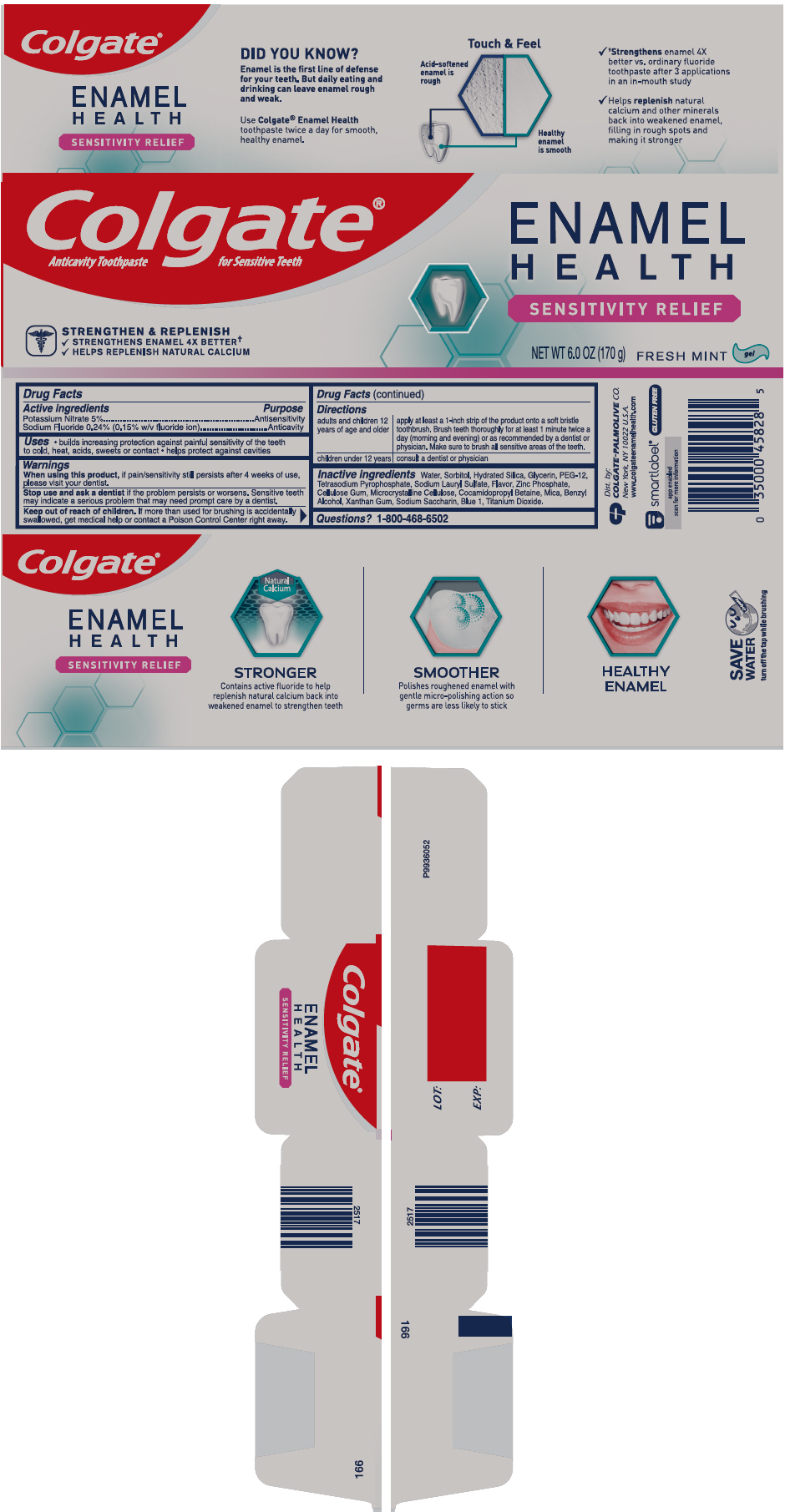
COLGATE ENAMEL HEALTH SENSITIVITY RELIEF- potassium nitrate and sodium fluoride gel, dentifrice
Colgate-Palmolive
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Colgate® Enamel Health™ Sensitivity Relief
Uses
- builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets or contact
- helps protect against cavities
Warnings
When using this product, if pain/sensitivity still persists after 4 weeks of use, please visit your dentist.
Directions
| adults and children 2 years of age and older | apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist or physician. Make sure to brush all sensitive areas of the teeth. |
| children under 12 years | consult a dentist or physician |
Inactive ingredients
water, sorbitol, hydrated silica, glycerin, PEG-12, tetrasodium pyrophosphate, sodium lauryl sulfate, flavor, microcrystalline cellulose, zinc phosphate, cellulose gum, cocamidopropyl betaine, benzyl alcohol, sodium saccharin, xanthan gum, mica, titanium dioxide, FD&C blue no. 1
| COLGATE ENAMEL HEALTH SENSITIVITY RELIEF
potassium nitrate and sodium fluoride gel, dentifrice |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Colgate-Palmolive (001344381) |
Revised: 10/2019
Document Id: 99e12e24-1c9e-44d5-bd5c-61e68e85479e
Set id: f9a1545d-1116-48fa-8403-69b9758020ad
Version: 9
Effective Time: 20191007
Colgate-Palmolive