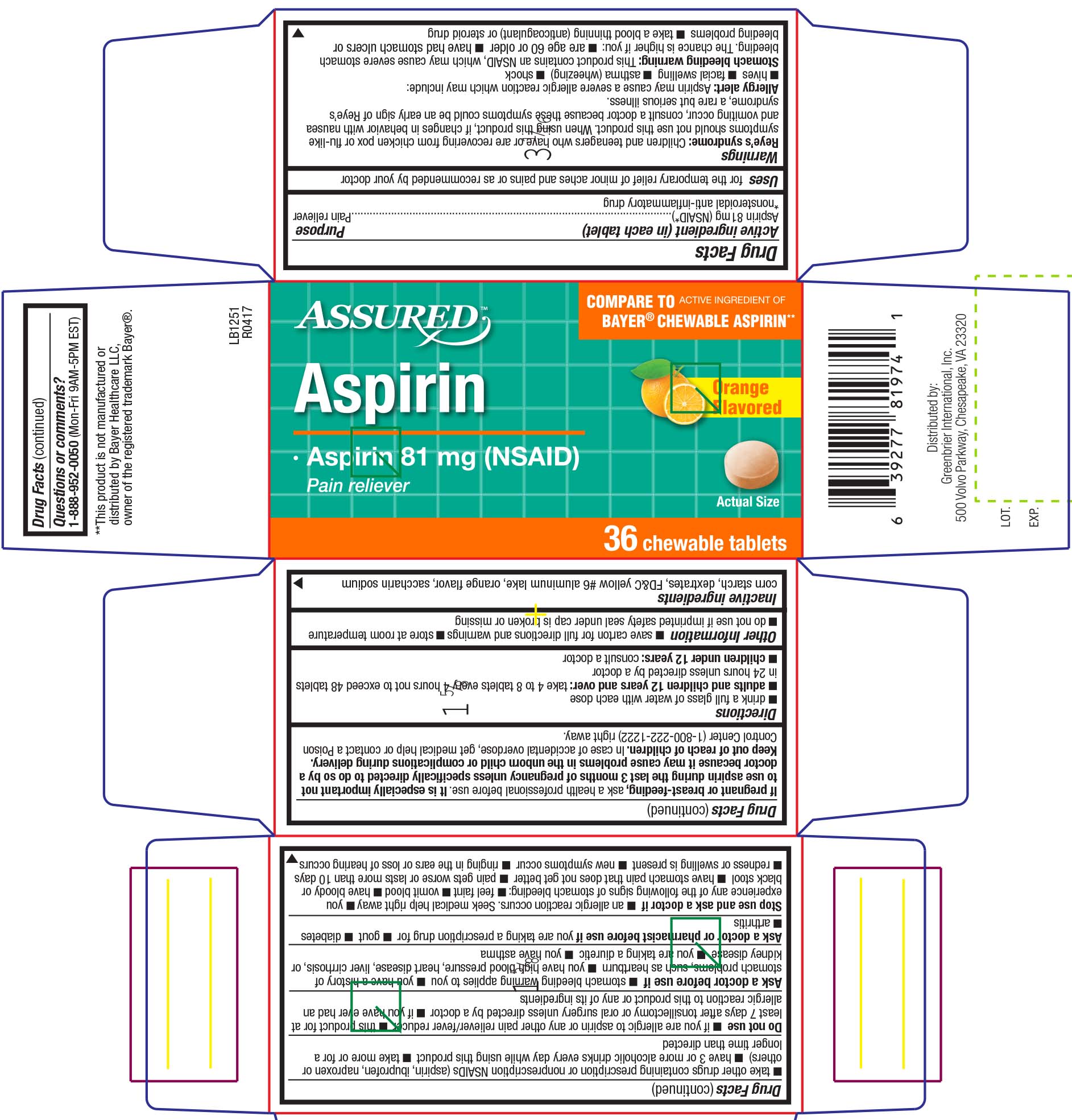
ASPIRIN- aspirin tablet, chewable
Greenbrier International
----------
288 - Aspirin 81mg
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use
- if you are allergic to aspirin or any other pain reliever/fever reducer
- this product for at least 7 days after tonsillectomy or oral surgery unless directed by a doctor
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
- you have asthma
Ask a doctor or pharmacist before use if
you are taking a prescription drug for
- gout
- diabetes
- arthritis
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away
you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stool
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- redness or swelling is present
- new symptoms occur
- ringing in the ears or loss of hearing occurs
If pregnant or breast-feeding
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless specifically directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Directions
- drink a full glass of water with each dose
- adults and children 12 years and over: take 4 to 8 tablets every 4 hours not to exceed 48 tablets in 24 hours unless directed by a doctor
- children under 12 years: consult a doctor
Other information
- save carton for full directions and warnings
- store at room temperature
- do not use if imprinted safety seal under cap is broken or missing
| ASPIRIN
aspirin tablet, chewable |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Greenbrier International (610322518) |