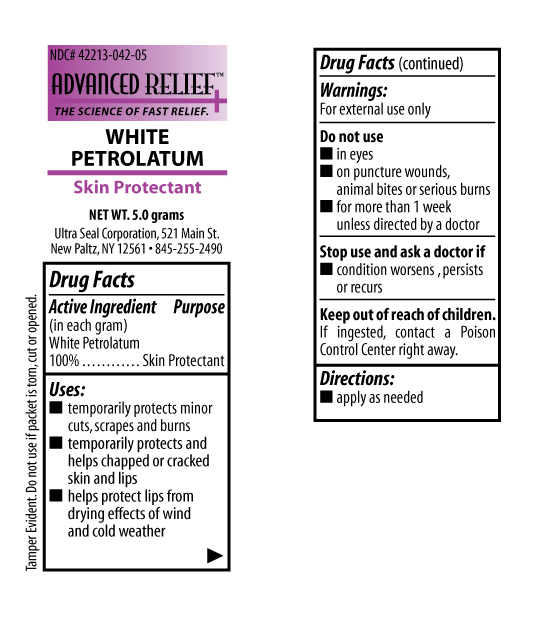
ADVANCED PETROLATUM- petrolatum ointment
Ultra Seal Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Temporarily protects minor cuts, scrapes, and burns. Temporarily protects and helps chapped or cracked skin and lips. Helps protect lips from drying effects fo wind and cold weather.
| ADVANCED PETROLATUM
petrolatum ointment |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Ultra Seal Corporation (085752004) |
| Registrant - Ultra Seal Corporation (085752004) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ultra Seal Corporation | 085752004 | pack(42213-042) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ultra Seal Corporation | 944090448 | pack(42213-042) | |
Revised: 12/2022
Document Id: f0fe66ff-926b-5b2a-e053-2995a90a90c4
Set id: f9164696-3128-4712-9276-e1d3e69dfd1b
Version: 6
Effective Time: 20221229
Ultra Seal Corporation