BYE, BYE VER- artemisia vulgaris, filix mas, tanacetum vulgare, teucrium marum, absinthium, cina, chenopodium anthelminticum, liquid
Apotheca Company
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Bye, Bye Ver
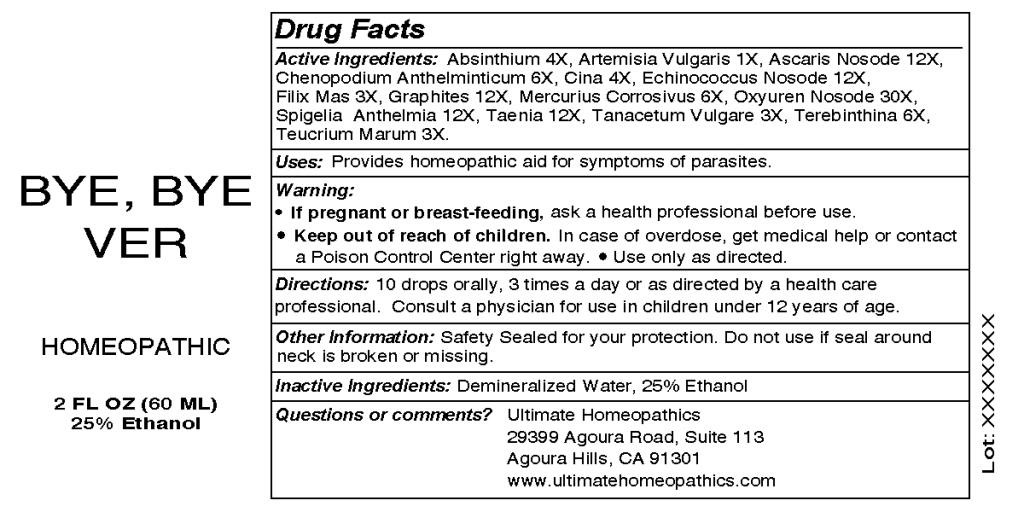
Active Ingredients: Artemisia vulgaris 1X, Filix Mas 3X, Tanacetum Vulgare 3X, Teucrium Marum 3X, Absinthium 4X, Cina 4X, Chenopodium Anthelminticum 6X, Mercurius Corrosivus 6X, Terebinthina 6X, Ascaris Nosode 12X, Echinococcus Nosode 12X, Graphites 12X, Spigelia Anthelmia 12X, Taenia 12X, Oxyuren Nosode 30X.
WARNING:
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Use only as directed.
OTHER INFORMATION: Safety Sealed for your protection. Do not use if seal around neck is broken or missing.
Directions: 10 drops orally, 3 times a day or as directed by a health care professional. Consult a physician for use in children under 12 years of age.
KEEP OUT OF REACH OF CHILDREN. In case of overdose, get medical help or contact a Poison Control Center right away.
| BYE, BYE VER
artemisia vulgaris, filix mas, tanacetum vulgare, teucrium marum, absinthium, cina, chenopodium anthelminticum, liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Apotheca Company (844330915) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(57520-0061) , api manufacture(57520-0061) , label(57520-0061) , pack(57520-0061) | |