Label: CROMOLYN SODIUM solution/ drops
- NDC Code(s): 53002-0140-1
- Packager: RPK Pharmaceuticals, Inc.
- This is a repackaged label.
- Source NDC Code(s): 61314-237
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated June 7, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Cromolyn Sodium Ophthalmic Solution USP, 4% is a clear, colorless, sterile solution intended for topical ophthalmic use.
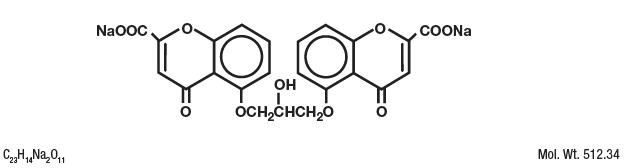
Cromolyn sodium is represented by the following structural formula:
Chemical Name: Disodium 5-5'-[(2-hydroxytrimethylene)dioxy]bis[4-oxo-4H-1-benzopyran-2-carboxylate]
Pharmacologic Category: Mast cell stabilizer.
EACH mL CONTAINS: Active: cromolyn sodium 40 mg (4%); Preservative: benzalkonium chloride 0.01%. Inactives: edetate disodium 0.1% and purified water. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH (4.0-7.0).
-
CLINICAL PHARMACOLOGY
In vitro and in vivo animal studies have shown that cromolyn sodium inhibits the degranulation of sensitized mast cells which occurs after exposure to specific antigens. Cromolyn sodium acts by inhibiting the release of histamine and SRS-A (slow-reacting substance of anaphylaxis) from the mast cell.
Another activity demonstrated in vitro is the capacity of cromolyn sodium to inhibit the degranulation of non-sensitized rat mast cells by phospholipase A and the subsequent release of chemical mediators. Another study showed that cromolyn sodium did not inhibit the enzymatic activity of released phospholipase A on its specific substrate.
Cromolyn sodium has no intrinsic vasoconstrictor, antihistaminic or anti-inflammatory activity.
Cromolyn sodium is poorly absorbed. When multiple doses of cromolyn sodium ophthalmic solution are instilled into normal rabbit eyes, less than 0.07% of the administered dose of cromolyn sodium is absorbed into the systemic circulation (presumably by way of the eye, nasal passages, buccal cavity and gastrointestinal tract). Trace amounts (less than 0.01%) of the cromolyn sodium dose penetrate into the aqueous humor and clearance from this chamber is virtually complete within 24 hours after treatment is stopped.
In normal volunteers, analysis of drug excretion indicates that approximately 0.03% of cromolyn sodium is absorbed following administration to the eye.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General
Patients may experience a transient stinging or burning sensation following application of Cromolyn Sodium Ophthalmic Solution USP, 4%.
The recommended frequency of administration should not be exceeded (see DOSAGE AND ADMINISTRATION).
Information for the Patient
Patients should be advised to follow the patient instructions listed on the Information for Patients sheet.
Users of contact lenses should refrain from wearing lenses while exhibiting the signs and symptoms of vernal keratoconjunctivitis, vernal conjunctivitis, or vernal keratitis. Do not wear contact lenses during treatment with Cromolyn Sodium Ophthalmic Solution USP, 4%.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies of cromolyn sodium in mice (12 months intraperitoneal administration at doses up to 150 mg/kg three days per week), hamsters (intraperitoneal administration at doses up to 52.6 mg/kg three days per week for 15 weeks followed by 17.5 mg/kg three days per week for 37 weeks), and rats (18 months subcutaneous administration at doses up to 75 mg/kg six days per week) showed no neoplastic effects. The average daily maximum dose levels administered in these studies were 192.9 mg/m2 for mice, 47.2 mg/m2 for hamsters and 385.8 mg/m2 for rats. These doses correspond to approximately 6.8, 1.7 and 14 times the maximum daily human dose of 28 mg/m2.
Cromolyn sodium showed no mutagenic potential in the Ames Salmonella/microsome plate assays, mitotic gene conversion in Saccharomycas cerevisiae and in an in vitro cytogenetic study in human peripheral lymphocytes.
No evidence of impaired fertility was shown in laboratory reproduction studies conducted subcutaneously in rats at the highest doses tested, 175 mg/kg/day (1050 mg/m2) in males and 100 mg/kg/day (600 mg/m2) in females. These doses are approximately 37 and 21 times the maximum daily human dose, respectively, based on mg/m2.
Teratogenic Effects
Pregnancy Category B. Reproduction studies with cromolyn sodium administered subcutaneously to pregnant mice and rats at maximum daily doses of 540 mg/kg (1620 mg/m2) and 164 mg/kg (984 mg/m2), respectively, and intravenously to rabbits at a maximum daily dose of 485 mg/kg (5820 mg/m2) produced no evidence of fetal malformation. These doses represent approximately 57, 35, and 205 times the maximum daily human dose, respectively, on a mg/m2 basis. Adverse fetal effects (increased resorption and decreased fetal weight) were noted only at the very high parenteral doses that produced maternal toxicity. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
-
ADVERSE REACTIONS
The most frequently reported adverse reaction attributed to the use of cromolyn sodium ophthalmic solution, on the basis of reoccurrence following readministration, is transient ocular stinging or burning upon instillation.
The following adverse reactions have been reported as infrequent events. It is unclear whether they are attributable to the drug:
Conjunctival injection; watery eyes; itchy eyes; dryness around the eye; puffy eyes; eye irritation; and styes.
Immediate hypersensitivity reactions have been reported rarely and include dyspnea, edema and rash.
-
DOSAGE AND ADMINISTRATION
The dose is 1 or 2 drops in each eye 4 to 6 times a day at regular intervals. One drop contains approximately 1.6 mg cromolyn sodium.
Patients should be advised that the effect of cromolyn sodium ophthalmic solution therapy is dependent upon its administration at regular intervals, as directed.
Symptomatic response to therapy (decreased itching, tearing, redness, and discharge) is usually evident within a few days, but longer treatment for up to six weeks is sometimes required. Once symptomatic improvement has been established, therapy should be continued for as long as needed to sustain improvement.
If required, corticosteroids may be used concomitantly with Cromolyn Sodium Ophthalmic Solution USP, 4%.
- HOW SUPPLIED
-
SPL UNCLASSIFIED SECTION
300049857-0821
Manufactured by
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 for
Sandoz Inc.
Princeton, NJ 08540
Printed in USA
August 2021
Information for the Patient
Cromolyn Sodium (KRO moe lin SOE dee um) Ophthalmic Solution USP, 4%
Sterile
It is important to use Cromolyn Sodium Ophthalmic Solution USP, 4% regularly, as directed by your physician.
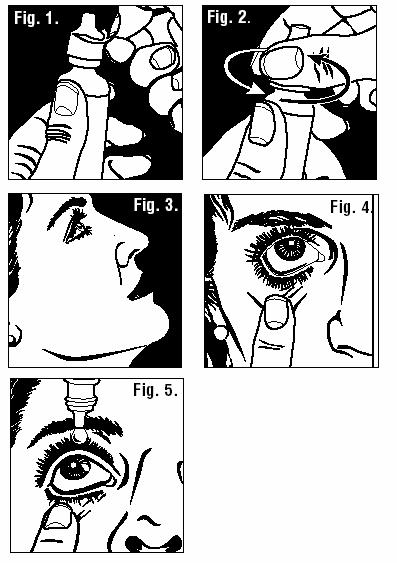
- 1.
- Thoroughly wash your hands.
- 2.
- Remove safety seal (Figure 1).
- 3.
- Remove cap (Figure 2).
- 4.
- Sit or stand comfortably, with your head tilted back (Figure 3).
- 5.
- Open eyes, look up, and draw the lower lid of your eye down gently with your index finger (Figure 4).
- 6.
- Hold the Cromolyn Sodium Ophthalmic Solution USP, 4% bottle upside down. Place dropper tip as close as possible to the lower eyelid and gently squeeze out the prescribed number of drops (Figure 5).
- 7.
- Do not touch the eye or eyelid with the dropper tip.
- 8.
- Blink a few times to make sure the eye is covered with the solution.
- 9.
- Close your eye and remove any excess solution with a clean tissue.
- 10.
- Repeat process in the other eye.
SPECIAL TIPS
- 1.
- Avoid placing Cromolyn Sodium Ophthalmic Solution USP, 4% directly on the cornea (the area just over the pupil), because it is especially sensitive. You will find the administration of the eye drops more comfortable if you place the drops just inside the lower eyelid as shown in Figure 5.
- 2.
- To avoid contamination of the solution, do not touch dropper tip to the eye, fingers, or any other surface. Replace cap after use. It is recommended that any remaining contents be discarded after the treatment period prescribed by your physician.
- 3.
- Store between 15°-30°C (59°-86°F). Protect from light - store in original carton.
- 4.
- Keep tightly closed and out of the reach of children.
- 5.
- Do not use with any other ocular medication unless directed by your physician. Do not wear contact lenses during treatment with Cromolyn Sodium Ophthalmic Solution USP, 4%.
Manufactured by
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 for
Sandoz Inc.
Princeton, NJ 08540
Printed in USA
August 2021
300049857-0821
- Cromolyn Na 4% Ophthalmic Solution
-
INGREDIENTS AND APPEARANCE
CROMOLYN SODIUM
cromolyn sodium solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53002-0140(NDC:61314-237) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CROMOLYN SODIUM (UNII: Q2WXR1I0PK) (CROMOLYN - UNII:Y0TK0FS77W) CROMOLYN SODIUM 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53002-0140-1 10 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075282 06/29/1999 Labeler - RPK Pharmaceuticals, Inc. (147096275) Establishment Name Address ID/FEI Business Operations RPK Pharmaceuticals, Inc. 147096275 RELABEL(53002-0140)