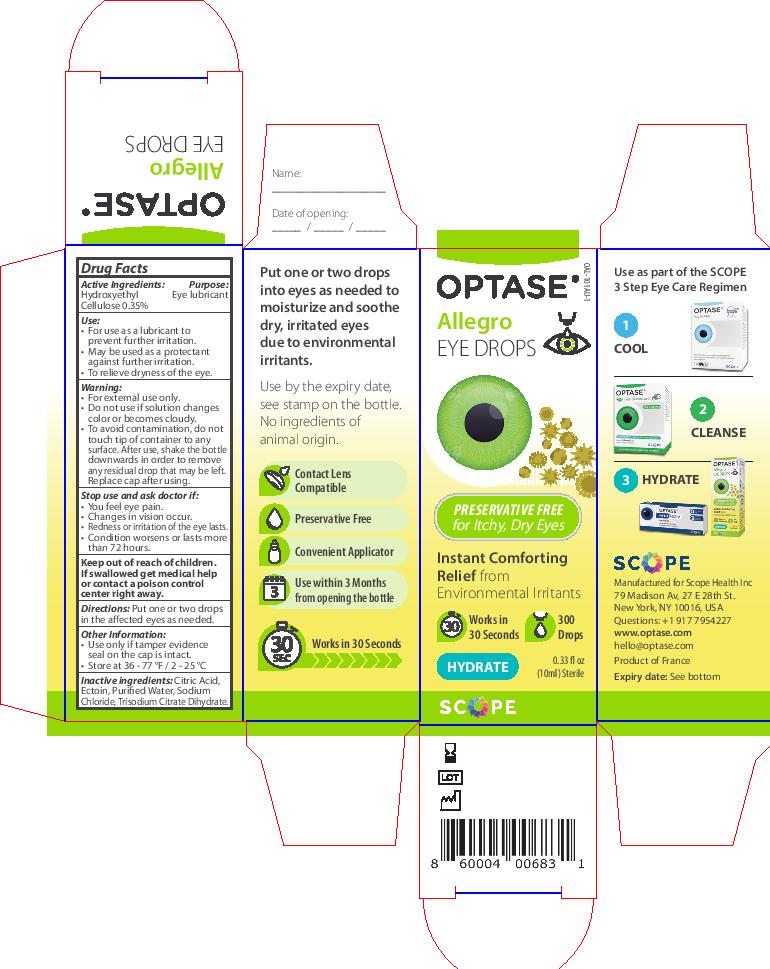
Label: OPTASE ALLEGRO- hydroxyethyl cellulose solution/ drops
- NDC Code(s): 72972-006-01
- Packager: Scope Health Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 19, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
WARNINGS
Warning
• For external use only.
• If solution changes color or
becomes cloudy, do not use.To avoid contamination, do
not touch tip to any surface.
After use, shake the bottle
downwards in order to remove
any residual drop that may be left.
Replace cap after using.
Stop use and ask doctor if:
Stop use and ask doctor if
• You feel eye pain.
• Changes in vision occur.
• Redness or irritation of the eye lasts.
• Condition worsens or lasts
more than 72 hours - INDICATIONS & USAGE
- Patient Information Leaflet
- PRINCIPAL DISPLAY PANEL
- Immediate Label
-
INGREDIENTS AND APPEARANCE
OPTASE ALLEGRO
hydroxyethyl cellulose solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72972-006 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROXYETHYL CELLULOSE (140 CPS AT 5%) (UNII: 8136Y38GY5) (HYDROXYETHYL CELLULOSE (140 CPS AT 5%) - UNII:8136Y38GY5) HYDROXYETHYL CELLULOSE (140 CPS AT 5%) 0.35 mg in 1 mL Inactive Ingredients Ingredient Name Strength ECTOINE (UNII: 7GXZ3858RY) SODIUM CHLORIDE (UNII: 451W47IQ8X) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72972-006-01 1 in 1 BOX 03/23/2023 1 10 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 03/23/2023 Labeler - Scope Health Inc (116778693) Registrant - Regulatory Matters Consulting (080711165)