Label: IDELVION- coagulation factor ix recombinant human kit
-
NDC Code(s):
69911-765-82,
69911-765-85,
69911-864-02,
69911-865-02, view more69911-866-02, 69911-867-02, 69911-869-02, 69911-874-01, 69911-875-01, 69911-876-01, 69911-877-01, 69911-879-01
- Packager: CSL Behring Lengnau AG
- Category: PLASMA DERIVATIVE
Drug Label Information
Updated June 30, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use IDELVION® safely and effectively. See full prescribing information for IDELVION.
IDELVION [Coagulation Factor IX (Recombinant), Albumin Fusion Protein]
Lyophilized Powder for Solution for Intravenous Injection
Initial U.S. Approval: 2016INDICATIONS AND USAGE
IDELVION, Coagulation Factor IX (Recombinant), Albumin Fusion Protein (rIX-FP), a recombinant DNA-derived coagulation Factor IX concentrate, is indicated in children and adults with Hemophilia B (congenital Factor IX deficiency) for:
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding
- Routine prophylaxis to reduce the frequency of bleeding episodes
Limitations of Use:
IDELVION is not indicated for immune tolerance induction in patients with Hemophilia B. (1)
DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
- Each single-dose vial of IDELVION is labeled with the actual Factor IX potency in international units (IU). (2.1)
- One IU of IDELVION per kg body weight is expected to increase the circulating activity of Factor IX as follows:
- Administer intravenously. Do not exceed infusion rate of 10 mL per minute. (2.3)
On-demand treatment and control of bleeding episodes and perioperative management:
- Dosage and duration of treatment with IDELVION depends on the severity of the Factor IX deficiency, the location and extent of bleeding, and the patient's clinical condition, age and recovery of Factor IX. (2.1)
- Determine the initial dose using the following formula:
Required Dose (IU) = Body Weight (kg) × Desired Factor IX rise (% of normal or IU/dL) × (reciprocal of recovery (IU/kg per IU/dL)) (2.1) - Adjust dose based on the patient's clinical condition and response. (2.1)
Routine prophylaxis:
DOSAGE FORMS AND STRENGTHS
IDELVION is available as a lyophilized powder in single-dose vials containing nominally 250, 500, 1000, 2000, or 3500 IU. (3)
CONTRAINDICATIONS
Do not use in patients who have had life-threatening hypersensitivity reactions to IDELVION or its components, including hamster proteins. (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity have been reported. Should symptoms of hypersensitivity reactions including anaphylaxis occur, discontinue IDELVION and administer appropriate treatment. (5.1)
- The formation of neutralizing antibodies (inhibitors) to Factor IX has been reported with IDELVION. If expected Factor IX plasma recovery in patient plasma is not attained, or if bleeding is not controlled with an appropriate dose, perform an assay that measures Factor IX inhibitor concentration. (5.2, 5.5)
- Thromboembolism (e.g., pulmonary embolism, venous thrombosis, and arterial thrombosis) may occur when using Factor IX-containing products. (5.3)
- Nephrotic syndrome has been reported following immune tolerance induction with Factor IX-containing products in hemophilia B patients with Factor IX inhibitors and a history of allergic reactions to Factor IX. (5.4)
- Factor IX activity assay results may vary with the type of activated partial thromboplastin time reagent used. (5.5)
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥1%) reported in clinical trials were headache, dizziness, hypersensitivity and rash. (6)
To report SUSPECTED ADVERSE REACTIONS, contact CSL Behring Pharmacovigilance Department at 1-866-915-6958 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pediatric: Higher dose per kilogram body weight or more frequent dosing may be needed. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Preparation and Reconstitution
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Neutralizing Antibodies
5.3 Thromboembolic Complications
5.4 Nephrotic Syndrome
5.5 Monitoring Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
IDELVION®, Coagulation Factor IX (Recombinant), Albumin Fusion Protein (rIX-FP), a recombinant DNA-derived coagulation Factor IX concentrate, is indicated in children and adults with Hemophilia B (congenital Factor IX deficiency) for:
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding
- Routine prophylaxis to reduce the frequency of bleeding episodes
-
2 DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
2.1 Dosage
- Each single-dose vial of IDELVION contains the recombinant Factor IX potency in international units (IU) that is stated on the carton and vial label.
- Dosage and duration of treatment with IDELVION depends on the severity of Factor IX deficiency, the location and extent of bleeding, and the patient's clinical condition, age and recovery of Factor IX.
- The calculation of the required dose of IDELVION is based on the empirical finding that one IU of IDELVION per kg body weight is expected to increase the circulating level of Factor IX by 1.3 IU/dL in patients ≥12 years of age and by 1 IU/dL in patients <12 years of age. The required dose of IDELVION for treatment of bleeding episodes is determined using the following formula:
Required Units (IU) = Body Weight (kg) × Desired Factor IX rise (% of normal or IU/dL) × (reciprocal of recovery (IU/kg per IU/dL)) OR Increase in Factor IX IU/dL (or % of normal) = Dose (IU) × Recovery (IU/dL per IU/kg)/body weight (kg) - Adjust the dose based on the individual patient's clinical condition and response.
On-demand Treatment and Control of Bleeding Episodes
A guide for dosing IDELVION for the on-demand treatment and control of bleeding episodes is provided in Table 1. Dosing should aim at maintaining a plasma Factor IX activity level at or above the plasma levels (in % of normal or IU/dL) outlined in Table 1.
Table 1. Dosing for On-demand Treatment and Control of Bleeding Episodes Type of Bleeding Episode Circulating Factor IX Activity Required
[% or (IU/dL)]Frequency of Dosing (hours) Duration of Therapy (days)* - *
- Adapted from the WFH Guidelines for the Management of Hemophilia.
Minor or Moderate
Uncomplicated hemarthrosis, muscle bleeding (except iliopsoas) or oral bleeding30-60 48-72 At least 1 day, until bleeding stops and healing is achieved. Single dose should be sufficient for majority of bleeds. Major
Life or limb threatening hemorrhage, deep muscle bleeding, including iliopsoas, intracranial, retropharyngeal60-100 48-72 7-14 days, until bleeding stops and healing is achieved. Maintenance dose weekly. Perioperative Management of Bleeding
A guide for dosing IDELVION for perioperative management of bleeding is provided in Table 2.
Table 2. Dosing for Perioperative Management of Bleeding Type of Surgery Circulating Factor IX Activity Required
[% or (IU/dL)]Frequency of Dosing (hours) Duration of Therapy (days)* - *
- Adapted from the WFH Guidelines for the Management of Hemophilia.
Minor
(including uncomplicated tooth extraction)50-80 48-72 At least 1 day, or until healing is achieved. Single dose should be sufficient for a majority of minor surgeries. Major
(including intracranial, pharyngeal, retropharyngeal, retroperitoneal)60-100
(initial level)48-72 7-14 days, or until bleeding stops and healing is achieved. Repeat dose every 48-72 hours for the first week or until healing is achieved. Maintenance dose 1-2 times per week. Routine Prophylaxis
For patients ≥12 years of age, the recommended dose is 25-40 IU IDELVION per kg body weight every 7 days. Patients who are well-controlled on a 7-day regimen may be switched to a 14-day interval at 50-75 IU IDELVION per kg body weight [see Clinical Studies (14)].
For patients <12 years of age, the recommended dose is 40-55 IU IDELVION per kg body weight every 7 days.
- A regimen may be further individually adjusted to less or more frequent dosing in adults.
2.2 Preparation and Reconstitution
The procedures below are provided as general guidelines for the preparation and reconstitution of IDELVION.
- Always work on a clean surface and wash your hands before performing the following procedures.
- Use aseptic technique during the reconstitution procedure.
- Reconstitute IDELVION using the diluent (Sterile Water for Injection) and transfer device (Mix2Vial) provided in the kit.
- To administer, you will also need a syringe and suitable needle (not provided).
- Ensure the vials of IDELVION and the Sterile Water for Injection are at room temperature before mixing.
- The reconstitution is performed as described below.
IDELVION Reconstitution Instructions
- Place the IDELVION vial, diluent vial, and Mix2Vial® transfer set on a flat surface.
- Remove flip caps from the IDELVION and Sterile Water for Injection (diluent) vials.
- Wipe the stoppers with the sterile alcohol swab provided and allow to dry prior to opening the Mix2Vial transfer set package.
- Open the Mix2Vial transfer set package by peeling away the lid (Fig. 1). Do not remove the device from the package.
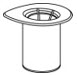
Fig. 1 - Place the diluent vial on a flat surface and hold the vial tightly. Grip the Mix2Vial transfer set together with the clear package and push the plastic spike at the blue end of the Mix2Vial transfer set firmly through the center of the stopper of the diluent vial (Fig. 2).
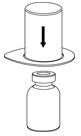
Fig. 2 - Carefully remove the clear package from the Mix2Vial transfer set. Do not remove the Mix2Vial transfer set or touch the exposed end of the device (Fig. 3).
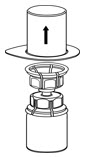
Fig. 3 - With the IDELVION vial placed firmly on a flat surface, invert the diluent vial with the Mix2Vial transfer set attached and push the plastic spike of the transparent adapter firmly through the center of the stopper of the IDELVION vial (Fig. 4). The diluent will automatically transfer into the IDELVION vial.
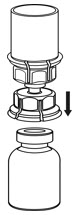
Fig. 4 - With the diluent and IDELVION vial still attached to the Mix2Vial transfer set, gently swirl the IDELVION vial to ensure that the powder is fully dissolved (Fig. 5). Do not shake the vial.
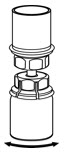
Fig. 5 - With one hand, grasp the IDELVION side of the Mix2Vial transfer set and with the other hand grasp the blue diluent-side of the Mix2Vial transfer set, and unscrew the set into two pieces (Fig. 6).
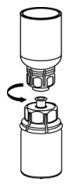
Fig. 6 - Draw air into an empty, sterile syringe. While the IDELVION vial is upright, screw the syringe to the Mix2Vial transfer set. Inject air into the IDELVION vial.
- While keeping the syringe plunger pressed, invert the system upside down and draw the concentrate into the syringe by pulling the plunger back slowly (Fig. 7).
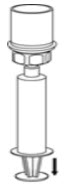
Fig. 7 - Disconnect the filled syringe by unscrewing it from the Mix2Vial transfer set (Fig. 8). The reconstituted solution should be a clear or yellow to colorless solution. Do not use if particulate matter or discoloration is observed.
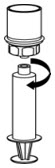
Fig. 8 - Use immediately or within 4 hours of reconstitution. Store reconstituted solution at room temperature. Do not refrigerate.
- If the dose requires more than one vial, use a separate, unused Mix2Vial transfer set and Sterile Water for Injection (diluent) vial for each product vial. Repeat steps 10-12 to pool the contents of the vials into one syringe.
2.3 Administration
For intravenous injection only.
- Do not mix or administer IDELVION in the same tubing or container with other medicinal products.
- Visually inspect the final solution for particulate matter and discoloration prior to administration, and whenever solution and container permit. Do not use if particulate matter or discoloration is observed.
- Attach the syringe containing the reconstituted IDELVION solution to a sterile infusion set and administer by intravenous injection. Adapt the infusion rate to the comfort level of each patient, not exceeding 10 mL per minute.
- Administer IDELVION at room temperature and within 4 hours of reconstitution. Discard any unused product.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
IDELVION is contraindicated in patients who have had life-threatening hypersensitivity reactions to IDELVION, or its components, including hamster proteins [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions have occurred. Early signs of hypersensitivity reactions, which can progress to anaphylaxis include angioedema, chest tightness, hypotension, generalized urticaria, wheezing, and dyspnea. If symptoms of hypersensitivity or anaphylaxis occur, immediately discontinue administration and initiate appropriate treatment.
IDELVION contains trace amounts of Chinese hamster ovary (CHO) proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
5.2 Neutralizing Antibodies
The formation of neutralizing antibodies (inhibitors) to Factor IX has been reported with IDELVION [see Adverse Reactions (6.3)]. Monitor all patients treated with IDELVION for the development of neutralizing antibodies (inhibitors) by appropriate clinical observations or laboratory tests. Perform an assay that measures Factor IX inhibitor concentration if expected plasma Factor IX activity levels are not attained, or if the bleeding is not controlled with an appropriate dose.
Patients with Factor IX inhibitors are at an increased risk of severe hypersensitivity reactions or anaphylaxis if re-exposed to Factor IX. Evaluate patients experiencing allergic reactions for the presence of an inhibitor and closely monitor patients with inhibitors for signs and symptoms of acute hypersensitivity reactions, particularly during early phases of exposure to the product [see Warnings and Precautions (5.1)].
5.3 Thromboembolic Complications
Thromboembolism (e.g., pulmonary embolism, venous thrombosis, and arterial thrombosis) may occur when using Factor IX-containing products. Because of the potential risk for thromboembolism with the use of Factor IX products, monitor for early signs of thromboembolism and consumptive coagulopathy when administering IDELVION to patients with liver disease, fibrinolysis, perioperative status, or risk factors for thromboembolic events or disseminated intravascular coagulation.
5.4 Nephrotic Syndrome
Nephrotic syndrome has been reported following attempted immune tolerance induction in hemophilia B patients with Factor IX inhibitors and a history of allergic reactions. The safety and efficacy of using IDELVION for immune tolerance induction have not been established.
5.5 Monitoring Laboratory Tests
- Monitor Factor IX plasma levels by a one-stage clotting assay to confirm that adequate Factor IX levels have been achieved and maintained [see Dosage and Administration (2.1)]. Factor IX activity assay results may vary with the type of activated partial thromboplastin time (aPTT) reagent used in the assay system.1 For example, kaolin-based aPTT reagents along with other reagents designed to exhibit low responsiveness to lupus anticoagulant2 have been shown to result in approximately 50% lower than expected recovery based on labeled potency.
- Consistent with similar findings for other recombinant Factor IX products, overestimation of Factor IX activity in spiked samples of IDELVION (mean overestimation 32%) occurred at low Factor IX levels with commonly used aPTT reagents.3
- Monitor patients for the development of inhibitors if expected Factor IX activity plasma levels are not attained, or if bleeding is not controlled with the recommended dose of IDELVION. Assays used to determine if a Factor IX inhibitor is present should be titered in Bethesda Units (BUs).
-
6 ADVERSE REACTIONS
The most common adverse reactions (incidence ≥1%) reported in clinical trials were headache, dizziness, hypersensitivity and rash.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
In five multicenter, prospective, open-label clinical trials with IDELVION, 114 previously treated patients (PTPs; exposed to a Factor IX-containing product for ≥100 exposure days (EDs)) received at least one infusion of IDELVION as part of on-demand treatment of bleeding episodes, perioperative management of major and minor surgical, dental, or other invasive procedures, routine prophylaxis, or pharmacokinetic evaluation. Twenty-seven children were <12 years of age, 9 adolescents were 12 to <18 years of age, and 78 adults were ≥18 to ≤65 years of age. A total of 16,326 injections were administered over a median of 1,543.5 days (range: 25 to 2,565 days).
Adverse reactions that occurred in >0.5% of subjects are listed in Table 3.
Table 3. Summary of Adverse Reactions in Previously Treated Patients MedDRA Standard
System Organ ClassAdverse Reaction Number of subjects
n (%), (N=114)Nervous system disorders Headache 2 (1.8) Dizziness 2 (1.8) Immune system disorders Hypersensitivity 1 (0.9) Skin and subcutaneous tissue disorders Rash 1 (0.9) Eczema 1 (0.9) Safety of IDELVION was also evaluated in a completed study with 12 previously untreated patients (PUPs). The median number of EDs was 50 (range: 22 to 146) per subject, a total of 8 subjects achieved at least 50 EDs, of whom 3 subjects achieved ≥100 EDs.
Adverse drug reactions were reported in 2 of 12 subjects treated with IDELVION. One subject (8.3%) in the PUP study developed a high titer of factor IX inhibitors (peak titer 188 BU/mL) and the same subject (8.3%) developed hypersensitivity. One additional subject (8.3%) in the PUP study developed rash.
6.2 Immunogenicity
All subjects were monitored for inhibitory and binding antibodies to rIX-FP (specifically rFIX, pdFIX and albumin), and binding antibodies to CHO host cell proteins at the following time points: at screening, at 2-4 weeks, 12 weeks following the first infusion of IDELVION, every 3 months thereafter (every 6 months for binding antibodies in children). No subjects developed non-neutralizing antibodies against factor IX, or antibodies to albumin and CHO protein at any of the time points following infusion of IDELVION. One case of inhibitor development to Factor IX was reported in the clinical study which evaluated previously untreated patients. No antibodies to Factor IX have been observed in IDELVION clinical trials which enrolled previously treated patients.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, it may be misleading to compare the incidence of antibodies to IDELVION in the studies described above with the incidence of antibodies in other studies or to other products.
6.3 Postmarketing Experience
The following adverse reaction has been identified during post-approval use of IDELVION. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: Factor IX inhibitor development
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with IDELVION use in pregnant women to inform on drug-associated risk. Animal reproduction studies have not been conducted using IDELVION. It is not known whether IDELVION can cause fetal harm or affect reproduction capacity when administered to a pregnant woman. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the excretion of IDELVION in human milk, the effect on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for IDELVION and any potential adverse effects on the breastfed infant from IDELVION or from the underlying maternal condition.
8.4 Pediatric Use
In clinical studies that included 34 previously treated patients (PTPs) <18 years old, the prophylactic administration with IDELVION every 7 days was successful in prevention of spontaneous bleeding episodes requiring treatment [see Clinical Studies (14)]. Of these, 7 subjects were ≥12 and <18 years old; 5 of these subjects switched to a 10- or 14-day prophylactic administration with IDELVION. There were no apparent differences in the safety profile in subjects <18 years as compared to adults [see Adverse Reactions (6.1)].
Compared to adults, incremental rIX-FP recovery appeared to be slightly lower and body weight-adjusted clearance appeared to be higher. Children may have higher Factor IX body weight-adjusted clearance, shorter half-life, and lower recovery. Higher dose per kilogram body weight or more frequent dosing may be needed in these patients [see Dosage and Administration (2.1) and Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
IDELVION, Coagulation Factor IX (Recombinant), Albumin Fusion Protein (rIX-FP) is a sterile, non-pyrogenic, lyophilized powder to be reconstituted with sterile Water for Injection (sWFI) for intravenous administration. IDELVION is available in single-dose vials containing nominally 250, 500, 1000, 2000, or 3500 IU of Factor IX formulated with sodium citrate, polysorbate 80, mannitol and sucrose. The actual amount of Factor IX activity in IU is labeled on each vial. After reconstitution of the lyophilized powder, all dosage strengths yield a clear, yellow to colorless solution. IDELVION contains no preservatives.
The active ingredient in IDELVION, recombinant human coagulation Factor IX albumin fusion protein, is a purified protein produced by recombinant DNA technology. It is generated by the genetic fusion of recombinant albumin to recombinant coagulation Factor IX. The genetic fusion of the cDNA of human albumin to the cDNA of human coagulation Factor IX enables the gene product to be expressed as a single recombinant protein designated as rIX-FP. The Factor IX portion of IDELVION is identical to the Thr148 allelic form of human plasma-derived Factor IX. The cleavable linker between the Factor IX and albumin moieties is derived from the endogenous activation peptide in native Factor IX. rIX-FP remains intact in the circulation until Factor IX is activated, whereupon albumin is cleaved from Factor IX, releasing activated Factor IX (FIXa) when it is needed for coagulation.
IDELVION is manufactured without the addition of proteins derived from human or animal source materials. IDELVION is a glycoprotein consisting of 1018 amino acids secreted by a genetically engineered Chinese hamster ovary (CHO) cell line. The CHO cell line secretes rIX-FP into a defined cell culture medium and the rIX-FP protein is purified by a process that does not require the use of a monoclonal antibody reagent. The manufacturing process incorporates three validated virus clearance steps, including virus inactivation by solvent/detergent treatment and virus removal by filtration.
The potency expressed in International Units is determined using an in vitro aPTT-based one-stage clotting assay against CSL Behring's manufacturing reference standard. This internal potency standard has been calibrated against the World Health Organization (WHO) International Standard for Factor IX concentrate by a one-stage clotting assay using synthetic silica and synthetic phospholipid-based reagents.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
IDELVION is a recombinant protein that temporarily replaces the missing coagulation Factor IX needed for effective hemostasis. IDELVION is comprised of genetically fused recombinant coagulation Factor IX and recombinant albumin. Fusion with recombinant albumin extends the half-life of Factor IX [see Description (11) and Clinical Pharmacology (12.3)].
12.2 Pharmacodynamics
The administration of IDELVION increases plasma levels of Factor IX and can temporarily correct the coagulation defect in patients.
12.3 Pharmacokinetics
Adults ≥18 years
The pharmacokinetic (PK) profiles of IDELVION were evaluated following intravenous injections of single doses of 25, 50 or 75 IU/kg. The PK parameters were based on plasma Factor IX activity measured by the one-stage clotting assay. Blood samples for PK analysis were collected prior to dosing and up to 336 hours (14 days) after dosing.
Table 4 provides the pharmacokinetic parameters following a single 25 IU/kg, 50 IU/kg, or 75 IU/kg dose of IDELVION.
Table 4. Pharmacokinetic Parameters (Arithmetic Mean, CV%) Following a Single 25 IU/kg, 50 IU/kg, or 75 IU/kg Dose of IDELVION PK Parameters rIX-FP
25 (IU/kg)
(N=7)rIX-FP
50 (IU/kg)
(N=47)rIX-FP
75 (IU/kg)
(N=8)IR = incremental recovery recorded 30 minutes after injection; AUC = area under the Factor IX activity time curve; t1/2 = half-life; MRT = mean residence time; CL = body weight adjusted clearance; Vss = body weight adjusted volume of distribution at steady state. - *
- = Estimated time to mean Factor IX activity maintained above the pre-specified percent (%)
IR (IU/dL)/(IU/kg) 1.65 (11) 1.30 (24) 1.08 (20) Cmax (IU/dL) 41.1 (13) 66.6 (27) 82.0 (20) AUC0-inf (h*IU/dL) 4658 (36) 7482 (28) 9345 (20) t1/2 (hours) 118 (38) 104 (25) 104 (18) MRT (hours) 153 (24) 143 (23) 145 (14) CL (mL/h/kg) 0.57 (31) 0.73 (27) 0.84 (20) Vss (dL/kg) 0.86 (32) 1.02 (28) 1.20 (23) Time to 1% Factor IX Activity (days)* 22 (25) 23 (19) 25 (13) Time to 3% Factor IX Activity (days)* 14 (19) 17 (19) 18 (13) Time to 5% Factor IX Activity (days)* 10 (25) 13 (20) 15 (13) IDELVION PK parameters following single or repeat dosing for up to 30 weeks were similar.
Subjects <18 years
Pharmacokinetic parameters of IDELVION were evaluated in 5 adolescents (12 to <18 years of age) and 27 children (0 to <12 years of age) in open-label, multi-center studies following a 50 IU/kg intravenous injection of IDELVION. The PK samples were collected prior to dosing and at multiple time points up to 336 hours (14 days) after dosing.
Table 5 summarizes the PK parameters calculated from the pediatric data of 32 subjects, 0 to <18 years of age. The parameters estimated were based on the plasma Factor IX activity over time profile. Compared to adults, incremental recovery is lower (15% to 27%) and body weight-adjusted clearance is higher (45% to 62%) in children.
Table 5. Pharmacokinetic Parameters (Arithmetic Mean, CV%) of IDELVION Following a Single 50 IU/kg Dose PK Parameters 0 to <6 years
(N=12)6 to <12 years
(N=15)12 to <18 years
(N=5)IR = incremental recovery recorded 30 minutes after injection; AUC = area under the Factor IX activity time curve; t1/2 = half-life; MRT = mean residence time; CL = body weight adjusted clearance; Vss = body weight adjusted volume of distribution at steady-state. - *
- = Estimated time to mean Factor IX activity maintained above the pre-specified percent (%)
IR (IU/dL)/(IU/kg) 0.95 (22) 1.06 (23) 1.11 (28) Cmax (IU/dL) 48.3 (19) 52.9 (23) 55.3 (28) AUC0-inf (h*IU/dL) 4583 (33) 5123 (31) 5347 (48) t1/2 (hours) 90 (13) 93 (21) 87 (36) MRT (hours) 123 (14) 129 (19) 119 (31) CL (mL/h/kg) 1.18 (28) 1.06 (29) 1.08 (39) Vss (dL/kg) 1.42 (24) 1.32 (20) 1.16 (14) Time to 1% Factor IX Activity (days) * 17 (29) 20 (22) 19 (37) Time to 3% Factor IX Activity (days) * 12 (32) 14 (22) 13 (38) Time to 5% Factor IX Activity (days) * 9 (32) 11 (26) 11 (38) -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Nonclinical studies evaluating the carcinogenic potential of IDELVION have not been conducted.
No macroscopic or microscopic pathologies in reproductive organs were observed in animals dosed every day for 28 days with 6.7 times the maximum recommended prophylactic clinical dose of 75 IU/kg IDELVION. No animal studies regarding impairment of fertility following IDELVION dosing were conducted.
-
14 CLINICAL STUDIES
The safety and efficacy of IDELVION were evaluated in a prospective, open-label, multicenter clinical study of 63 male previously treated patients (PTPs) with hemophilia B (≤2% endogenous Factor IX activity) who received at least one infusion of IDELVION as part of on-demand treatment and control of bleeding episodes, perioperative management of major and minor surgical, dental, or other invasive procedures, routine prophylaxis once every 7-, 10- or 14-day intervals, or pharmacokinetic evaluation. Subjects were aged 12 to 61 years; including 7 adolescent subjects aged 12 to 17. Subjects were treated for up to 27 months.
On-demand Treatment and Control of Bleeding Episodes
A total of 358 bleeding events were treated with IDELVION. Among them, 204 (57%) bleeding events were spontaneous, 140 (39%) events were traumatic and 14 (4%) events were unknown. In addition, a total of 267 (75%) episodes were joint bleeding events.
Overall treatment efficacy was assessed for each bleeding episode by the investigator based on a 4-point scale of excellent, good, moderate, or poor/no response. The efficacy of IDELVION for the on-demand treatment and control of bleeding episodes is summarized in Table 6.
Table 6. Efficacy* of IDELVION for the On-demand Treatment and Control of Bleeding Episodes Number of Bleeding Episodes Requiring Treatment (n = 358) - *
- Excellent: Pain relief and/or unequivocal improvement in objective signs of bleeding and no additional infusion required in order to achieve hemostasis; Good: Definite pain relief and/or improvement in signs of bleeding at approximately 8 hours after the first infusion, but may require a second infusion; Moderate: Probable or slight beneficial effect, and requires more than two infusions to achieve hemostasis; Poor/no response: No improvement at all or condition worsens, additional hemostatic intervention is required. Responses evaluated at approximately 24 hours after treatment.
Number of injections to treat bleeding episodes 1 injection, n (%) 335 (94) 2 injections, n (%) 18 (5) >2 injections, n (%) 5 (1.4) Assessment of Efficacy* Excellent or Good, n (%) 337 (94) Moderate, n (%) 9 (2.5) Poor/no response, n (%) 1 (0.3) Perioperative Management of Bleeding
In the two effectiveness studies and the extension study, a total of 20 subjects (5-59 years of age) received IDELVION for perioperative management of 29 surgical procedures. Dose was individualized based on subject's PK and clinical response to treatment. These included orthopedic surgeries (n=14), a mastectomy with liposuction (n=1), a hemorrhoidectomy (n=2), rhinoplasty, submucosal resection and inferior turbinectomy (n=1), circumcision (n=3), tooth extractions (n=5), embolism of scrotal varicocele (n=1), excision of pigmented nevus (n=1) and endoscopic mucosal resection (n=1).
A single preoperative bolus was used in 97% (n=28) of surgeries. Hemostatic efficacy was rated as excellent or good for all surgeries at all time points for which hemostatic response was reported, except for one evaluation that was rated moderate at the 48-hour assessment. During the 14-day postoperative period, patients received between 0 and 11 infusions and FIX consumption was between 0 and 444.1 IU/kg. Six patients undergoing surgery were discharged from the surgical treatment site on the same day.
Routine Prophylaxis
Of the 63 subjects treated with IDELVION, twenty-three PTPs received IDELVION only for the treatment of bleeding episodes during the first 6 months of the study. Nineteen of these PTPs switched to once weekly prophylaxis with additional median duration of 10 months.
Based on the analysis of the 19 subjects treated with IDELVION for on-demand therapy and weekly prophylaxis, the median annualized spontaneous bleeding rate (AsBR) during prophylaxis treatment was 0.7 (range: 0 to 4.2), as compared to 15.4 (range: 2 to 39.5) during on-demand treatment. Based on the Poisson model, prophylaxis treatment with IDELVION resulted in a 93% reduction in the annualized spontaneous bleeding rate.
The median annualized bleeding rate (total ABR – spontaneous and traumatic) during prophylaxis treatment was 1.6; (range: 0 to 21.1), as compared to 19.2; (range: 2 to 46.1) during on demand treatment. Based on the Poisson model, prophylaxis treatment with IDELVION resulted in an 88% reduction in the annualized bleeding rate.
Table 7. Comparison of Annualized Bleeding Rates for Subjects Treated for On-demand Therapy and Weekly Prophylaxis Bleeding Episode Etiology On-demand (n=19)* Weekly Prophylaxis
(n=19)*Percent Reduction with Prophylaxis
(n=19)*IQR=interquartile range, defined for 25th percentile and 75th percentile; SD=standard deviation; Subjects evaluable for efficacy are subjects who received at least one dose of on-demand treatment, and one dose of prophylaxis treatment. Spontaneous Mean (SD) 14.6 (8.42) 0.9 (1.17) 93.5 (8.03) Median 15.4 0.7 95.9 IQR 8.0, 18.0 0, 1.6 89.0, 100 Range 2.0, 39.5 0, 4.2 75.2, 100 Total† Mean (SD) 20.8 (9.19) 2.9 (4.79) 88.0 (14.06) Median 19.2 1.6 90.9 IQR 16.7, 25.8 0, 4.3 81.2, 100 Range 2.0, 46.1 0, 21.1 54.3, 100 Forty subjects received weekly routine prophylaxis. Thirty-seven subjects completed 6 months of once weekly prophylaxis. Of these, 21 subjects switched to a 14-day interval with 50-75 IU/kg of IDELVION with a median duration of 12.7 months. Prior to switching, these 21 subjects were well controlled with IDELVION (did not require dose adjustments or experience spontaneous bleeding 1 month prior to switch and were maintained on a prophylaxis dose of ≤40 IU/kg every 7 days).
The median AsBRs for the 21 subjects treated with weekly and 14-day prophylaxis were zero (range: 0 to 4.5) and zero (range: 0 to 7.3), respectively (summarized in Table 8). In addition, the median AsBRs for the 7 subjects treated with weekly and 10-day prophylaxis were zero (range: 0 to 0) and zero (range: 0 to 0.9), respectively.
Table 8. Comparison of Annualized Spontaneous Bleeding Rate (AsBR) by Prophylaxis Regimen for Subjects in the Clinical Trial Bleeding Episode Etiology Weekly Prophylaxis
(n=21)*14 day Prophylaxis
(n=21)*IQR = interquartile range; SD = Standard deviation - *
- Based on a matched pairs design
Spontaneous Mean (SD) 0.28 (1.01) 1.07 (2.1) Median 0 0 IQR 0, 0 0, 1 Range 0, 4.5 0, 7.3 Routine Prophylaxis and Control of Bleeding in PTPs <12 Years
Safety, efficacy, and pharmacokinetics of IDELVION have been evaluated in previously treated pediatric patients (PTPs) ranging from one (1) to <12 years of age. A total of 27 males, 12 patients <6 years of age and 15 patients between 6 to <12 years of age, received IDELVION for prophylaxis and control of bleeding episodes. All 27 subjects received weekly prophylaxis treatment with IDELVION for a mean time on study of 13.1 months (range: 9, 18 months).
The routine weekly regimen with rIX-FP was effective in the prophylaxis of bleeding episodes. The median (Q1, Q3) ABR (total bleeding episodes, including untreated) and annualized spontaneous bleeding rate (AsBR) during prophylaxis treatment in the efficacy population were 3.41 [1.18,7.44] and 0 [0, 1.34] bleeding episodes/subject/year, respectively. The mean ABR was 4.55 bleeding episodes/year/subject and the mean AsBR was 0.79 based on the total of both treated and untreated bleeding episodes. The target joints (defined as 3 or more spontaneous bleeds into a single joint within a consecutive 6-months period) reported in 3 subjects prior to study entry were resolved during the study. All subjects maintained a weekly routine prophylaxis regimen throughout.
Of the 106 bleeding episodes, the majority (94;89%) was treated with single injection, 103 (97%) were treated with 1-2 injections. Hemostatic efficacy at resolution of a bleed was rated excellent or good in 96% of all treated bleeding episodes.
-
15 REFERENCES
- Wilmot HV, Hogwood J, Gray E. Recombinant factor IX: discrepancies between one-stage clotting and chromogenic assays. Haemophilia 2014, 1-7.
- Fritsma GA, Dembitzer FR, Randhawa A, et al. Recommendations for Appropriate Activated Partial Thromboplastin Time Reagent Selection and Utilization. Am J Clin Pathol 2012;137:904-908.
- Sommer JM, Buyue Y, Bardan S, Peters RT, Jiang H, Kamphaus G, Gray E, Pierce GF. Comparative field study: impact of laboratory assay variability on the assessment of recombinant factor IX Fc fusion protein (rFIXFc) activity. Thrombosis and Haemostasis 2014, 112(5):932-40.
-
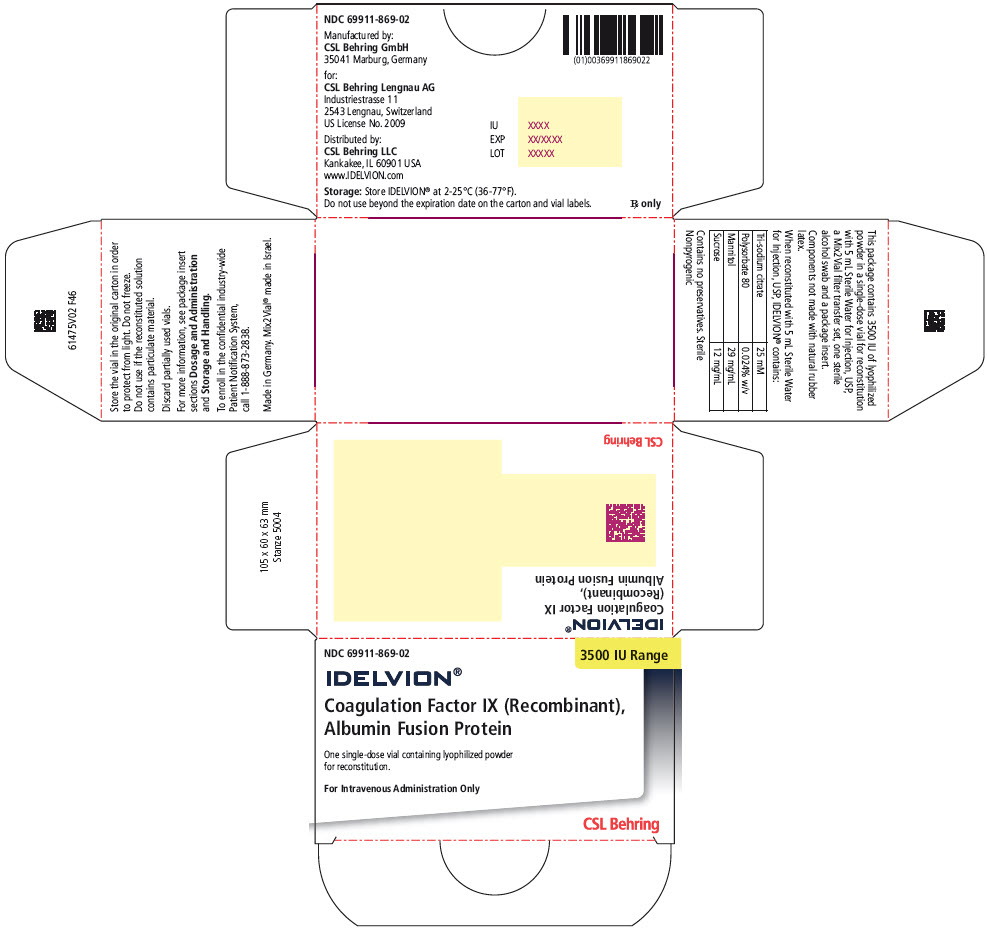
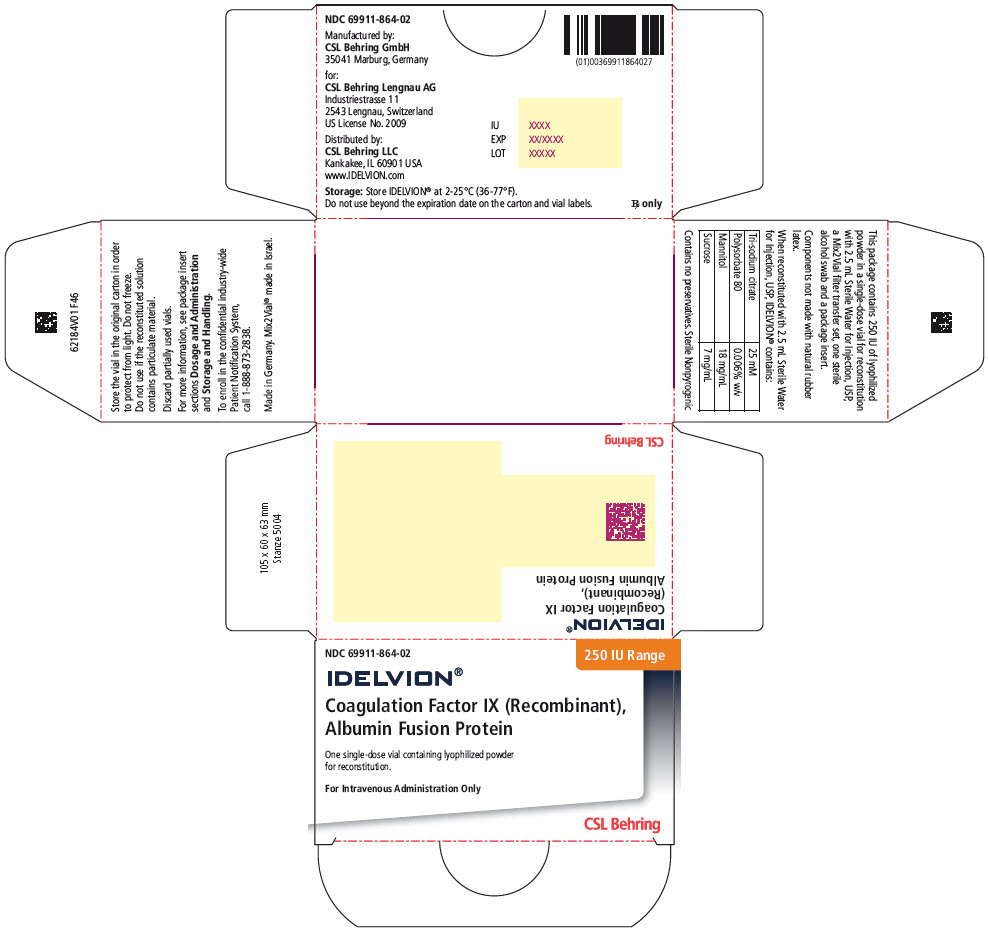
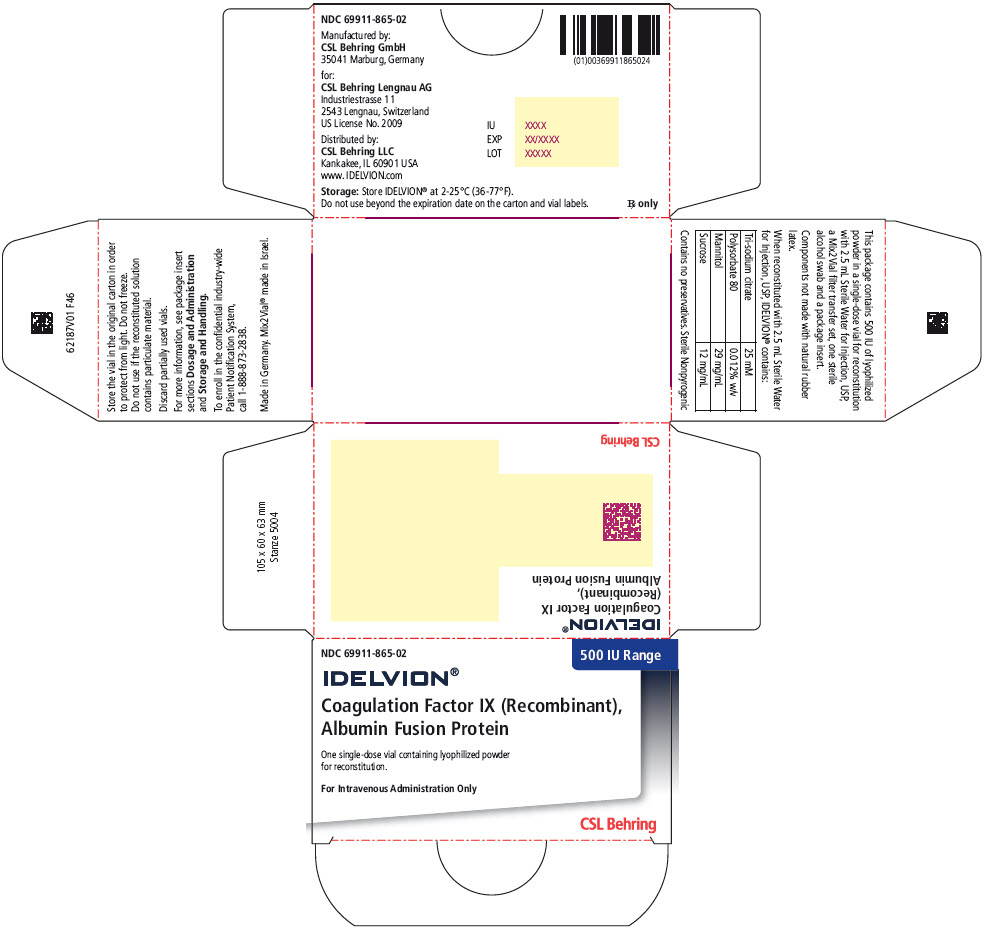
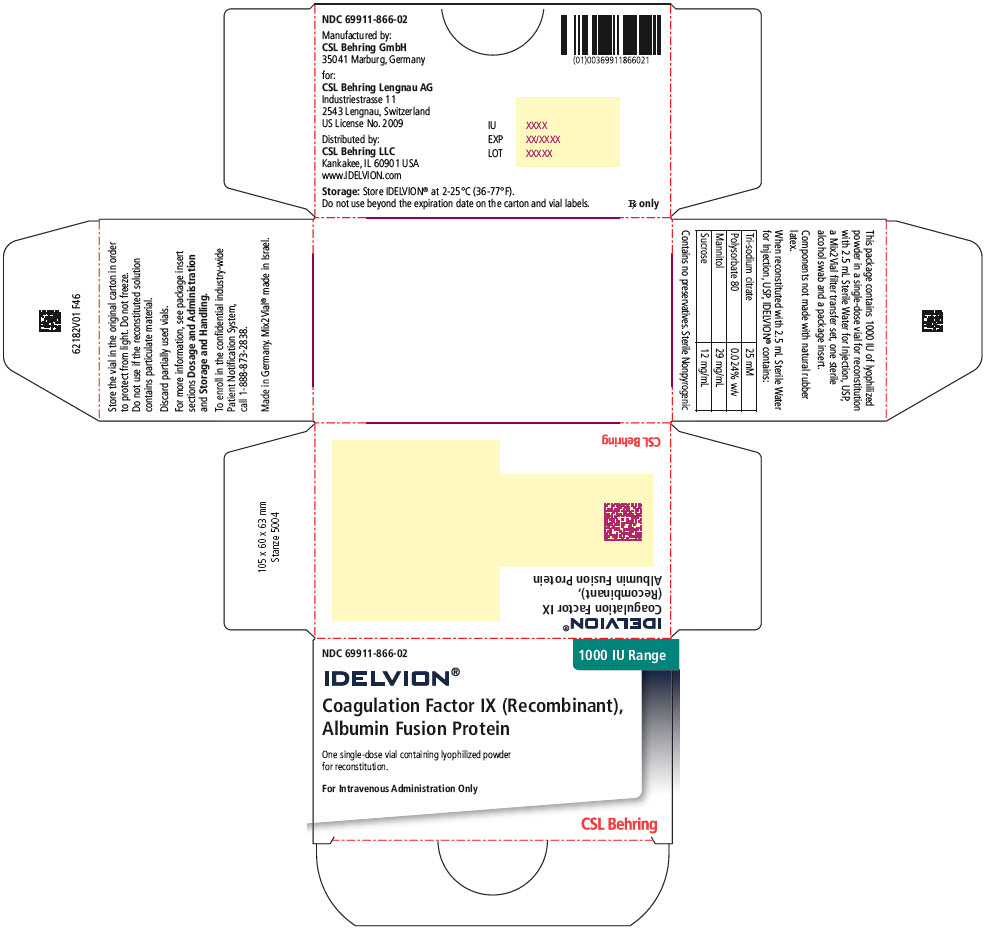
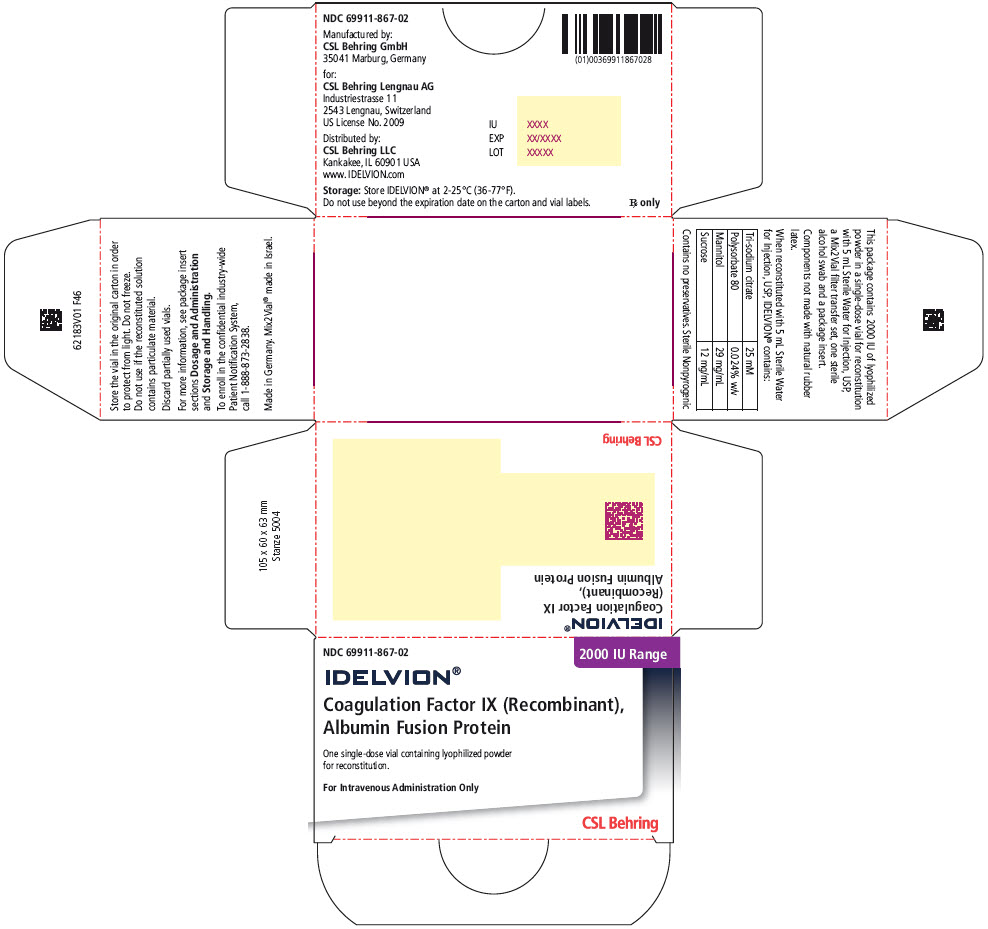
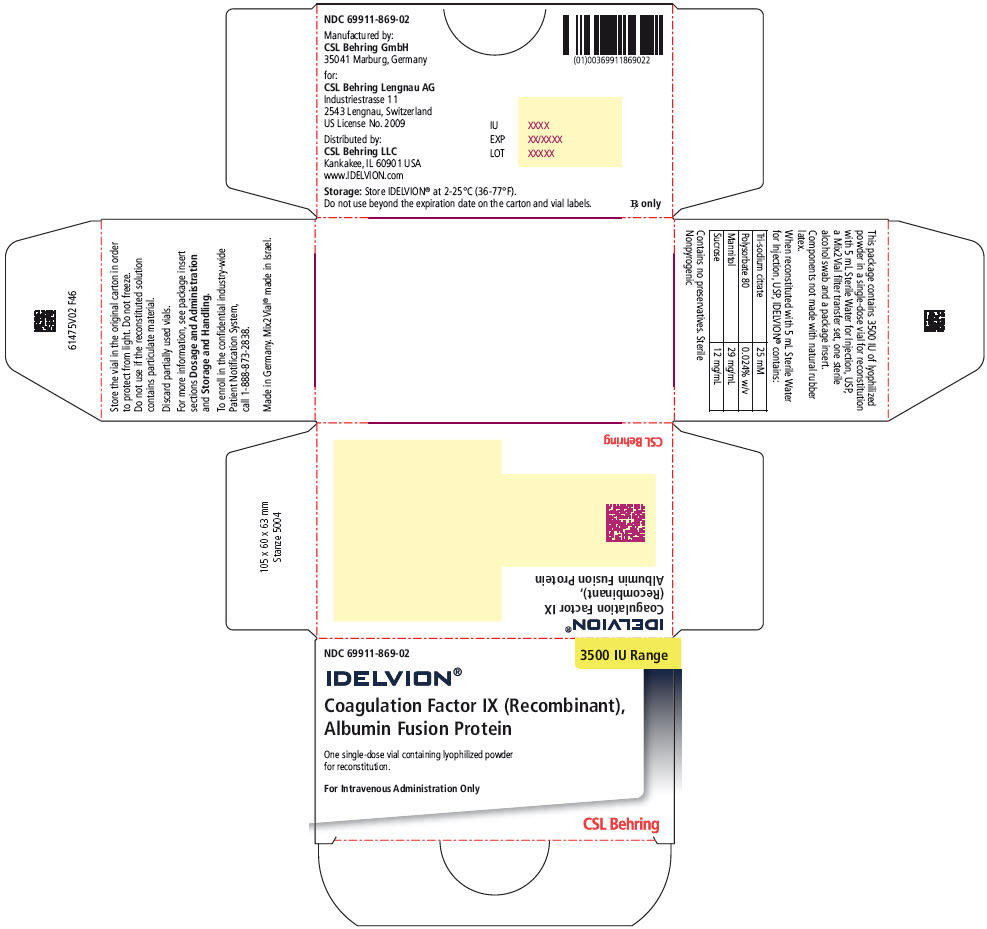
16 HOW SUPPLIED/STORAGE AND HANDLING
IDELVION is supplied as a lyophilized powder in single-dose vials containing the labeled amount of Factor IX activity, expressed in international units (IU).
IDELVION is packaged with 2.5 mL (for reconstitution of 250, 500 or 1000 IU vials) or 5 mL (for reconstitution of 2000 or 3500 IU vials) of Sterile Water for Injection, USP, one Mix2Vial filter transfer set, and one sterile alcohol swab. Components are not made with natural rubber latex.
Table 9. How Supplied Nominal Strength
(International Units)Fill Size
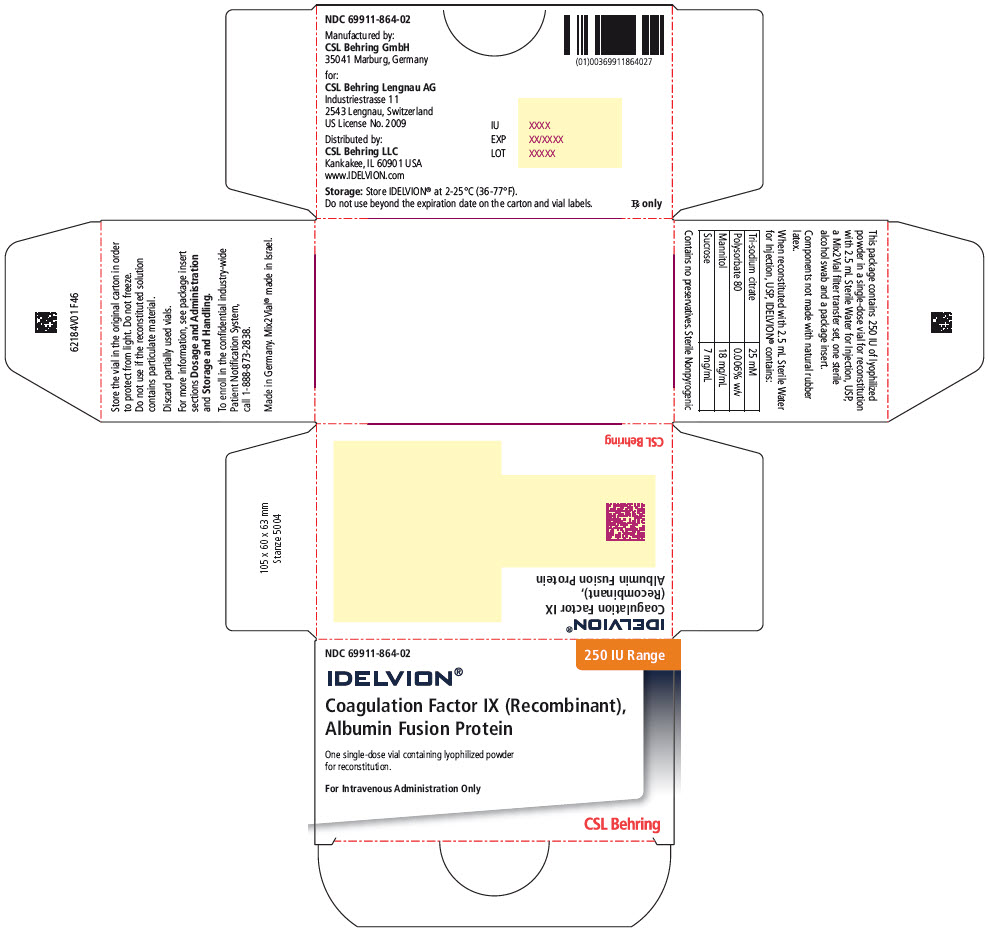
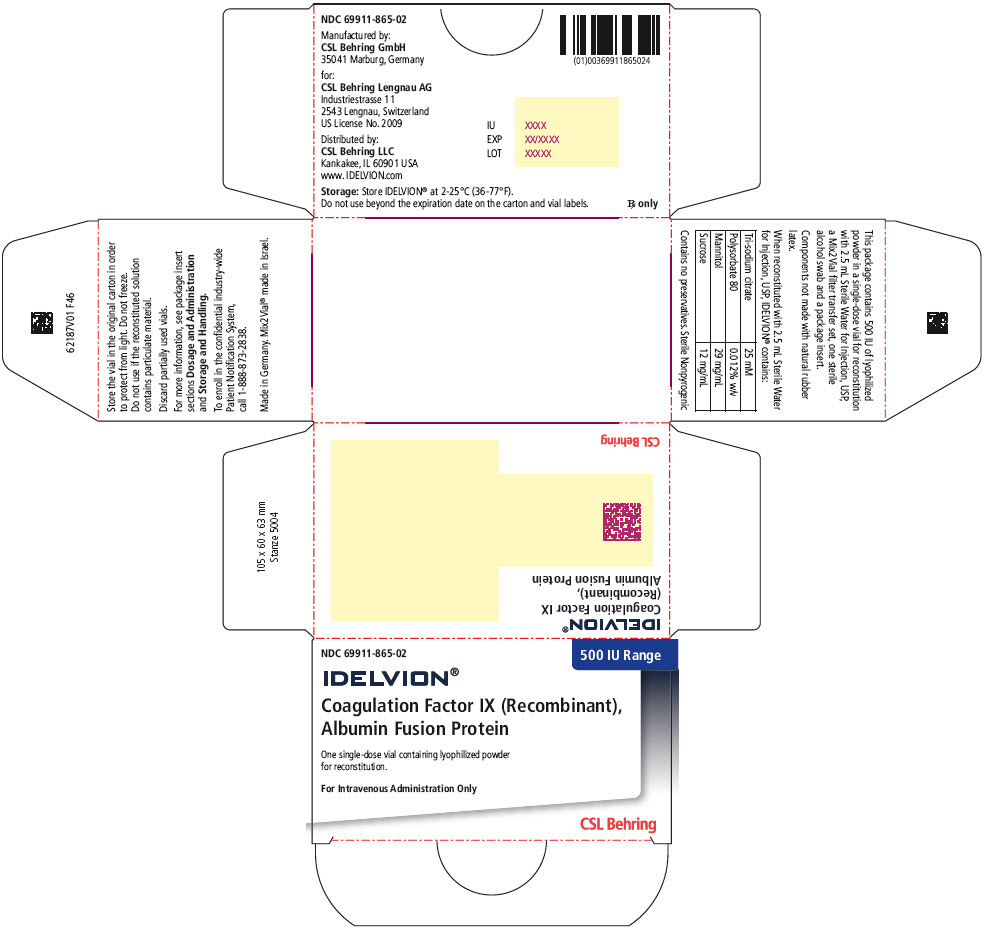
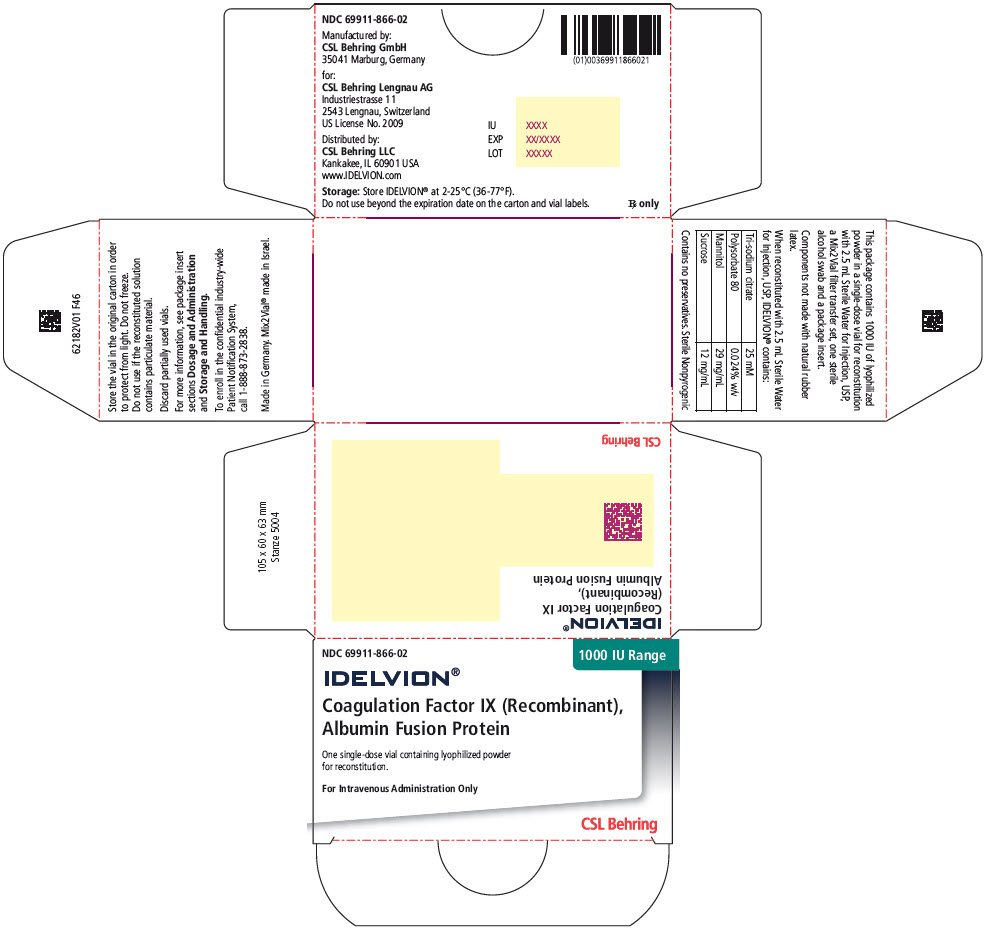
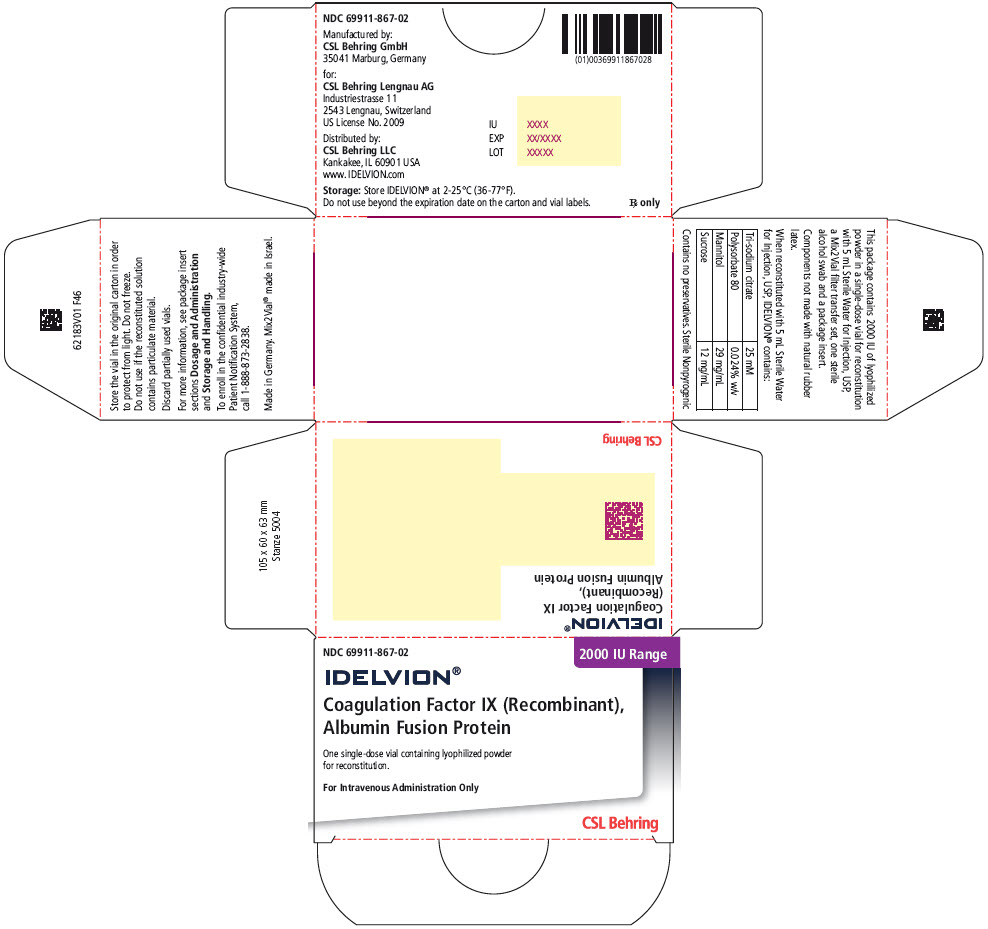
Color IndicatorKit NDC 250 Orange 69911-864-02 500 Blue 69911-865-02 1000 Green 69911-866-02 2000 Purple 69911-867-02 3500 Yellow 69911-869-02 Storage and Handling
- Store IDELVION in its package to protect from light.
- Store the IDELVION package in the refrigerator or at room temperature 2-25°C (36 to 77°F). Do not freeze.
- Do not use IDELVION or the Sterilized Water for Injection diluent beyond the expiration date printed on the carton and vial labels.
-
17 PATIENT COUNSELING INFORMATION
- Advise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Advise patients to report any adverse reactions or problems following IDELVION administration to their healthcare provider.
- Inform patients of the early signs and symptoms of hypersensitivity or allergic reactions (including hives, generalized urticaria, chest tightness, wheezing, and hypotension). Instruct patients to discontinue use of IDELVION and contact their healthcare provider and/or seek immediate emergency care if these symptoms occur [see Warnings and Precautions (5.1)].
- Advise patients to contact their healthcare provider or hemophilia treatment center for further treatment and/or assessment if they experience a lack of clinical response to Factor IX replacement therapy, as in some cases this may be a manifestation of an inhibitor [see Warnings and Precautions (5.2)].
-
SPL UNCLASSIFIED SECTION
Manufactured by:
CSL Behring GmbH
35041 Marburg, Germanyfor:
CSL Behring Lengnau AG
Biotech Innovation Park
2543 Lengnau, Switzerland
US License No. 2009Distributed by:
CSL Behring LLC
Kankakee, IL 60901 USAIDELVION® is manufactured under a license by Novozymes Biopharma A/S, Bagsvaerd, Denmark.
Mix2Vial® is a registered trademark of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceutical Services, Inc.
-
FDA-Approved Patient Labeling
IDELVION® (eye del' vee on),
Coagulation Factor IX (Recombinant), Albumin Fusion ProteinThis leaflet summarizes important information about IDELVION. Please read it carefully before using IDELVION. This information does not take the place of talking with your healthcare provider, and it does not include all of the important information about IDELVION. If you have any questions after reading this, ask your healthcare provider.
What is IDELVION?
IDELVION, Coagulation Factor IX (Recombinant), Albumin Fusion Protein is an injectable medicine used to replace clotting factor (Factor IX) that is missing in people with hemophilia B (also called congenital Factor IX deficiency or Christmas disease). Hemophilia B is an inherited bleeding disorder that prevents blood from clotting normally. IDELVION is used in children and adults with hemophilia B to control and prevent bleeding episodes. Your healthcare provider may give you IDELVION when you have surgery. IDELVION can reduce the number of bleeding episodes when used regularly (prophylaxis).
Who should not use IDELVION?
You should not use IDELVION if you:
- have had life-threatening hypersensitivity reactions to IDELVION
- are allergic to hamster proteins
- are allergic to any ingredients in IDELVION
Tell your healthcare provider if you have had an allergic reaction to any Factor IX product prior to using IDELVION.
Tell your healthcare provider if you are pregnant or breast-feeding because IDELVION may not be right for you.
What should I tell my healthcare provider before using IDELVION?
You should tell your healthcare provider if you:
- have or had any medical problems
- take any medicines, including prescription and non-prescription medicines, such as over-the-counter medicines, supplements, or herbal remedies
- have any allergies, including allergies to hamster proteins
- are breastfeeding. It is not known if IDELVION passes into your milk and if it can harm your baby.
- are pregnant or planning to become pregnant. It is not known if IDELVION may harm your unborn baby.
- have been told that you have inhibitors to Factor IX (because IDELVION may not work for you).
How should I administer IDELVION?
- IDELVION is given directly into the bloodstream.
- IDELVION should be administered as ordered by your healthcare provider.
- You should be trained on how to do injections by your healthcare provider or hemophilia treatment center. Many people with hemophilia B learn to inject IDELVION by themselves or with the help of a family member. See the step-by-step guide (Instructions for Use) provided at the end of this leaflet for directions on mixing and infusing IDELVION.
- Your healthcare provider will tell you how much IDELVION to use based on your weight, the severity of your hemophilia B, and where you are bleeding.
- You may need to have blood tests done after getting IDELVION to be sure that your blood level of Factor IX is high enough to clot your blood.
- Call your healthcare provider right away if your bleeding does not stop after taking IDELVION.
What are the possible side effects of IDELVION?
Allergic and hypersensitivity reactions have been reported with IDELVION. Call your healthcare provider right away and stop treatment if you get a rash or hives, itching, tightness of the chest or throat, difficulty breathing, light-headedness, dizziness, nausea, or decrease in blood pressure.
Your body can make antibodies, called inhibitors, against Factor IX which may stop IDELVION from working properly. Your healthcare provider may need to test your blood for inhibitors from time to time.
IDELVION may increase the risk of forming abnormal blood clots in your body, especially if you have risk factors for developing blood clots. Call your healthcare provider if you have chest pain, difficulty breathing, leg tenderness or swelling.
The most common side effects of IDELVION are headache and dizziness.
This is not the only side effect possible with IDELVION. To learn more, talk to your healthcare provider or pharmacist. Tell your healthcare provider about any side effect that bothers you or does not go away.
What are the IDELVION dosage strengths?
IDELVION comes in five different dosage strengths: 250, 500, 1000, 2000, or 3500 IU. The actual strength of IDELVION is printed on the carton and vial label. The labeling of the five dosage strengths are color-coded as provided in Table 1 below.
Table 1. IDELVION Dosage Strengths Fill Size Color Indicator Nominal Strength Orange 250 IU Blue 500 IU Green 1000 IU Purple 2000 IU Yellow 3500 IU Always check the actual dosage strength printed on the label to make sure you are using the strength prescribed by your healthcare provider.
How should I store IDELVION?
- Store vial in original carton to protect from light.
- Store at 2-25°C (36-77°F). Do not freeze.
- Do not use IDELVION after the expiration date printed on the label.
- The reconstituted product (after mixing dry product with diluent) must be used within 4 hours and cannot be stored or refrigerated. Discard any IDELVION left in the vial at the end of your infusion.
What else should I know about IDELVION?
- Do not use IDELVION for a condition for which it is not prescribed.
- Do not share IDELVION with other people, even if they have the same symptoms that you have.
-
Instructions for Use
For intravenous use after reconstitution only.
Do not attempt to do an intravenous injection unless you have been taught how by your healthcare provider or hemophilia center.
Always follow the specific instructions given by your healthcare provider. The steps listed below are general guidelines for using IDELVION. If you are unsure of the instructions, call your healthcare provider before using IDELVION. Call your healthcare provider right away if bleeding is not controlled after using IDELVION. Your healthcare provider will prescribe the dose that you should take. You may need to have blood tests from time to time. Talk to your healthcare provider before traveling. Dispose of all unused solution, empty vial(s), and other used medical supplies in an appropriate medical waste container.
- Always work on a clean, flat surface and wash your hands before performing the mixing (reconstitution) procedures.
- Mix (reconstitute) IDELVION using the diluent (Sterile Water for Injection) and transfer device (Mix2Vial) provided in the kit.
- To give an injection, you will also need a syringe and suitable needle (not provided).
- If a package is opened or damaged, do not use and contact your healthcare provider.
- Do not use IDELVION beyond the expiration date on the vial label and carton.
- Look at the mixed (reconstituted) solution. Do not use IDELVION if the reconstituted solution is cloudy, contains any particles, or is discolored.
- Each vial of IDELVION is for single-dose only. Contains no preservatives. Discard partially used vials.
- Ensure the vials of IDELVION and the Sterile Water for Injection are at room temperature before mixing.
- Follow the reconstitution instructions as described below.
IDELVION Reconstitution Instructions
- Place the IDELVION vial, diluent vial, and Mix2Vial® transfer set on a flat surface.
- Remove flip caps from the IDELVION and Sterile Water for Injection (diluent) vials.
- Wipe the stoppers with the sterile alcohol swab provided and allow to dry prior to opening the Mix2Vial transfer set package.
- Open the Mix2Vial transfer set package by peeling away the lid (Fig. 1). Do not remove the device from the package.
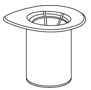
Fig. 1 - Place the diluent vial on a flat surface and hold the vial tightly. Grip the Mix2Vial transfer set together with the clear package and push the plastic spike at the blue end of the Mix2Vial transfer set firmly through the center of the stopper of the diluent vial (Fig. 2).
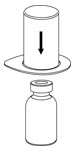
Fig. 2 - Carefully remove the clear package from the Mix2Vial transfer set. Do not remove the Mix2Vial transfer set or touch the exposed end of the device (Fig. 3).
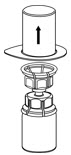
Fig. 3 - With the IDELVION vial placed firmly on a flat surface, invert the diluent vial with the Mix2Vial transfer set attached and push the plastic spike of the transparent adapter firmly through the center of the stopper of the IDELVION vial (Fig. 4). The diluent will automatically transfer into the IDELVION vial.
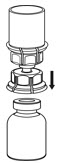
Fig. 4 - With the diluent and IDELVION vial still attached to the Mix2Vial transfer set, gently swirl the IDELVION vial to ensure that the powder is fully dissolved (Fig. 5). Do not shake the vial.
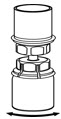
Fig. 5 - With one hand, grasp the IDELVION side of the Mix2Vial transfer set and with the other hand grasp the blue diluent-side of the Mix2Vial transfer set, and unscrew the set into two pieces (Fig. 6).
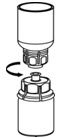
Fig. 6 - Draw air into an empty, sterile syringe. While the IDELVION vial is upright, screw the syringe to the Mix2Vial transfer set. Inject air into the IDELVION vial.
- While keeping the syringe plunger pressed, invert the system upside down and draw the concentrate into the syringe by pulling the plunger back slowly (Fig. 7).
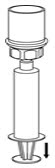
Fig. 7 - Disconnect the filled syringe by unscrewing it from the Mix2Vial transfer set (Fig. 8). The reconstituted solution should be a clear or yellow to colorless solution. Do not use if particulate matter or discoloration is observed.
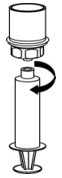
Fig. 8 - Use immediately or within 4 hours of reconstitution. Keep solution at room temperature. Do not refrigerate after reconstitution.
- If the dose requires more than one vial, use a separate, unused Mix2Vial transfer set and Sterile Water for Injection (diluent) vial for each product vial. Repeat steps 10-12 to pool the contents of the vials into one syringe.
- Record treatment – Remove the peel-off portion of the label from each vial used and affix it to the patient's treatment diary/log book.
Administration (intravenous injection)
- Do not mix IDELVION in the same tubing or container with other medicinal products.
- Attach the syringe containing the reconstituted IDELVION solution to a sterile infusion set and give an injection as directed by your healthcare provider or hemophilia treatment center.
- Administer intravenously. Do not exceed infusion rate 10 mL per minute.
Resources at CSL Behring available to the patient:
For Adverse Reaction Reporting contact:
CSL Behring Pharmacovigilance Department at 1-866-915-6958Contact CSL Behring to receive more product information:
Patient Support Hotline at 1-800-676-4266For more information, visit www.IDELVION.com
Manufactured by:
CSL Behring GmbH
35041 Marburg, Germanyfor:
CSL Behring Lengnau AG
Biotech Innovation Park
2543 Lengnau, Switzerland
US License No. 2009Distributed by:
CSL Behring LLC
Kankakee, IL 60901 USAIDELVION® is manufactured under a license by Novozymes Biopharma A/S, Bagsvaerd, Denmark.
Mix2Vial® is a registered trademark of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceutical Services, Inc.
Revised: 6/2023
- PRINCIPAL DISPLAY PANEL - 250 IU Range Kit Carton
- PRINCIPAL DISPLAY PANEL - 500 IU Range Kit Carton
- PRINCIPAL DISPLAY PANEL - 1000 IU Range Kit Carton
- PRINCIPAL DISPLAY PANEL - 2000 IU Range Kit Carton
- PRINCIPAL DISPLAY PANEL - 3500 IU Range Kit Carton
-
INGREDIENTS AND APPEARANCE
IDELVION
coagulation factor ix recombinant human kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:69911-864 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-864-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 2.5 mL Part 2 1 VIAL, SINGLE-USE 2.5 mL Part 1 of 2 COAGULATION FACTOR IX RECOMBINANT HUMAN
coagulation factor ix recombinant human injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:69911-874 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALBUTREPENONACOG ALFA (UNII: A57KX1VL5P) (ALBUTREPENONACOG ALFA - UNII:A57KX1VL5P) ALBUTREPENONACOG ALFA 250 [iU] in 2.5 mL Inactive Ingredients Ingredient Name Strength Sodium Citrate, Unspecified Form (UNII: 1Q73Q2JULR) Polysorbate 80 (UNII: 6OZP39ZG8H) Mannitol (UNII: 3OWL53L36A) Sucrose (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-874-01 2.5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 03/04/2016 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC:69911-765 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) 2.5 mL in 2.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-765-82 2.5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 03/04/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 03/04/2016 IDELVION
coagulation factor ix recombinant human kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:69911-865 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-865-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 2.5 mL Part 2 1 VIAL, SINGLE-USE 2.5 mL Part 1 of 2 COAGULATION FACTOR IX RECOMBINANT HUMAN
coagulation factor ix recombinant human injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:69911-875 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALBUTREPENONACOG ALFA (UNII: A57KX1VL5P) (ALBUTREPENONACOG ALFA - UNII:A57KX1VL5P) ALBUTREPENONACOG ALFA 500 [iU] in 2.5 mL Inactive Ingredients Ingredient Name Strength Sodium Citrate, Unspecified Form (UNII: 1Q73Q2JULR) Polysorbate 80 (UNII: 6OZP39ZG8H) Mannitol (UNII: 3OWL53L36A) Sucrose (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-875-01 2.5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 03/04/2016 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC:69911-765 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) 2.5 mL in 2.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-765-82 2.5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 03/04/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 03/04/2016 IDELVION
coagulation factor ix recombinant human kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:69911-866 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-866-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 2.5 mL Part 2 1 VIAL, SINGLE-USE 2.5 mL Part 1 of 2 COAGULATION FACTOR IX RECOMBINANT HUMAN
coagulation factor ix recombinant human injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:69911-876 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALBUTREPENONACOG ALFA (UNII: A57KX1VL5P) (ALBUTREPENONACOG ALFA - UNII:A57KX1VL5P) ALBUTREPENONACOG ALFA 1000 [iU] in 2.5 mL Inactive Ingredients Ingredient Name Strength Sodium Citrate, Unspecified Form (UNII: 1Q73Q2JULR) Polysorbate 80 (UNII: 6OZP39ZG8H) Mannitol (UNII: 3OWL53L36A) Sucrose (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-876-01 2.5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 03/04/2016 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC:69911-765 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) 2.5 mL in 2.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-765-82 2.5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 03/04/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 03/04/2016 IDELVION
coagulation factor ix recombinant human kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:69911-867 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-867-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 5 mL Part 2 1 VIAL, SINGLE-USE 5 mL Part 1 of 2 COAGULATION FACTOR IX RECOMBINANT HUMAN
coagulation factor ix recombinant human injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:69911-877 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALBUTREPENONACOG ALFA (UNII: A57KX1VL5P) (ALBUTREPENONACOG ALFA - UNII:A57KX1VL5P) ALBUTREPENONACOG ALFA 2000 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength Sodium Citrate, Unspecified Form (UNII: 1Q73Q2JULR) Polysorbate 80 (UNII: 6OZP39ZG8H) Mannitol (UNII: 3OWL53L36A) Sucrose (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-877-01 5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 03/04/2016 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC:69911-765 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-765-85 5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 03/04/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 03/04/2016 IDELVION
coagulation factor ix recombinant human kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:69911-869 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-869-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 5 mL Part 2 1 VIAL, SINGLE-USE 5 mL Part 1 of 2 COAGULATION FACTOR IX RECOMBINANT HUMAN
coagulation factor ix recombinant human injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:69911-879 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALBUTREPENONACOG ALFA (UNII: A57KX1VL5P) (ALBUTREPENONACOG ALFA - UNII:A57KX1VL5P) ALBUTREPENONACOG ALFA 3500 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength Sodium Citrate, Unspecified Form (UNII: 1Q73Q2JULR) Polysorbate 80 (UNII: 6OZP39ZG8H) Mannitol (UNII: 3OWL53L36A) Sucrose (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-879-01 5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 05/30/2018 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC:69911-765 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69911-765-85 5 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 05/30/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125582 05/30/2018 Labeler - CSL Behring Lengnau AG (480217014) Establishment Name Address ID/FEI Business Operations CSL Behring GmbH 326530474 MANUFACTURE(69911-864, 69911-865, 69911-866, 69911-867, 69911-869) Establishment Name Address ID/FEI Business Operations CSL Behring LLC 058268293 MANUFACTURE(69911-864, 69911-865, 69911-866, 69911-867, 69911-869)