DYAZIDE- hydrochlorothiazide and triamterene capsule
GlaxoSmithKline LLC
----------
DYAZIDE
(hydrochlorothiazide/triamterene)
Capsules
DESCRIPTION
Each capsule of DYAZIDE (hydrochlorothiazide and triamterene) for oral use, with opaque red cap and opaque white body, contains hydrochlorothiazide 25 mg and triamterene 37.5 mg, and is imprinted with the product name DYAZIDE and SB. Hydrochlorothiazide is a diuretic/antihypertensive agent and triamterene is an antikaliuretic agent.
Hydrochlorothiazide is slightly soluble in water. It is soluble in dilute ammonia, dilute aqueous sodium hydroxide, and dimethylformamide. It is sparingly soluble in methanol.
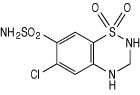
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide 1,1-dioxide, and its structural formula is:
At 50°C, triamterene is practically insoluble in water (less than 0.1%). It is soluble in formic acid, sparingly soluble in methoxyethanol, and very slightly soluble in alcohol.
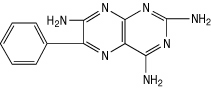
Triamterene is 2, 4, 7-triamino-6-phenylpteridine and its structural formula is:
Inactive ingredients consist of benzyl alcohol, cetylpyridinium chloride, D&C Red No. 33, FD&C Yellow No. 6, gelatin, glycine, lactose, magnesium stearate, microcrystalline cellulose, povidone, polysorbate 80, sodium starch glycolate, titanium dioxide, and trace amounts of other inactive ingredients.
Capsules of DYAZIDE meet Drug Release Test 3 as published in the current USP monograph for Triamterene and Hydrochlorothiazide Capsules.
CLINICAL PHARMACOLOGY
DYAZIDE is a diuretic/antihypertensive drug product that combines natriuretic and antikaliuretic effects. Each component complements the action of the other. The hydrochlorothiazide component blocks the reabsorption of sodium and chloride ions, and thereby increases the quantity of sodium traversing the distal tubule and the volume of water excreted. A portion of the additional sodium presented to the distal tubule is exchanged there for potassium and hydrogen ions. With continued use of hydrochlorothiazide and depletion of sodium, compensatory mechanisms tend to increase this exchange and may produce excessive loss of potassium, hydrogen, and chloride ions. Hydrochlorothiazide also decreases the excretion of calcium and uric acid, may increase the excretion of iodide, and may reduce glomerular filtration rate. The exact mechanism of the antihypertensive effect of hydrochlorothiazide is not known.
The triamterene component of DYAZIDE exerts its diuretic effect on the distal renal tubule to inhibit the reabsorption of sodium in exchange for potassium and hydrogen ions. Its natriuretic activity is limited by the amount of sodium reaching its site of action. Although it blocks the increase in this exchange that is stimulated by mineralocorticoids (chiefly aldosterone), it is not a competitive antagonist of aldosterone and its activity can be demonstrated in adrenalectomized rats and patients with Addison’s disease. As a result, the dose of triamterene required is not proportionally related to the level of mineralocorticoid activity but is dictated by the response of the individual patients and the kaliuretic effect of concomitantly administered drugs. By inhibiting the distal tubular exchange mechanism, triamterene maintains or increases the sodium excretion and reduces the excess loss of potassium, hydrogen, and chloride ions induced by hydrochlorothiazide. As with hydrochlorothiazide, triamterene may reduce glomerular filtration and renal plasma flow. Via this mechanism, it may reduce uric acid excretion although it has no tubular effect on uric acid reabsorption or secretion. Triamterene does not affect calcium excretion. No predictable antihypertensive effect has been demonstrated for triamterene.
Duration of diuretic activity and effective dosage range of the hydrochlorothiazide and triamterene components of DYAZIDE are similar. Onset of diuresis with DYAZIDE takes place within 1 hour, peaks at 2 to 3 hours, and tapers off during the subsequent 7 to 9 hours.
DYAZIDE is well absorbed.
Upon administration of a single oral dose to fasted normal male volunteers, mean pharmacokinetic parameters were determined (Table 1).
|
AUC(0-48) ng*h/mL (± SD) |
Cmax ng/mL (± SD) |
Median Tmax h |
Ae Mg (± SD) |
|
|
Triamterene |
148.7 (87.9) |
46.4 (29.4) |
1.1 |
2.7 (1.4) |
|
Hydroxytriamterene |
1,865 (471) |
720 (364) |
1.3 |
19.7 (6.1) |
|
Hydrochlorothiazide |
834 (177) |
135.1 (35.7) |
2.0 |
14.3 (3.8) |
a AUC(0-48), Cmax, Tmax, and Ae represent area under the plasma concentration versus time plot, maximum plasma concentration, time to reach Cmax, and amount excreted in urine over 48 hours.
A capsule of DYAZIDE is bioequivalent to a single entity 25-mg hydrochlorothiazide tablet and 37.5-mg triamterene capsule used in the double-blind clinical trial below (see Clinical Trials).
In a limited study involving 12 subjects, coadministration of DYAZIDE with a high-fat meal resulted in: (1) an increase in the mean bioavailability of triamterene by about 67% (90% confidence interval = 0.99, 1.90), p-hydroxytriamterene sulfate by about 50% (90% confidence interval = 1.06, 1.77), hydrochlorothiazide by about 17% (90% confidence interval = 0.90, 1.34); (2) increases in the peak concentrations of triamterene and p-hydroxytriamterene; and (3) a delay of up to 2 hours in the absorption of the active constituents.
CLINICAL TRIALS
A placebo-controlled, double-blind trial was conducted to evaluate the efficacy of DYAZIDE. This trial demonstrated that DYAZIDE (25 mg hydrochlorothiazide/37.5 mg triamterene) was effective in controlling blood pressure while reducing the incidence of hydrochlorothiazide-induced hypokalemia. This trial involved 636 patients with mild to moderate hypertension controlled by hydrochlorothiazide 25 mg daily and who had hypokalemia (serum potassium <3.5 mEq/L) secondary to the hydrochlorothiazide. Patients were randomly assigned to 4 weeks’ treatment with once-daily regimens of 25 mg hydrochlorothiazide plus placebo, or 25 mg hydrochlorothiazide combined with one of the following doses of triamterene: 25 mg, 37.5 mg, 50 mg, or 75 mg.
Blood pressure and serum potassium were monitored at baseline and throughout the trial. All 5 treatment groups had similar mean blood pressure and serum potassium concentrations at baseline (mean systolic blood pressure range: 137 ± 14 mmHg to 140 ± 16 mmHg; mean diastolic blood pressure range: 86 ± 9 mmHg to 88 ± 8 mmHg; mean serum potassium range: 2.3 to 3.4 mEq/L with the majority of patients having values between 3.1 and 3.4 mEq/L).
While all triamterene regimens reversed hypokalemia, at Week 4 the 37.5-mg regimen proved optimal compared with the other tested regimens. On this regimen, 81% of the patients had a significant (P<0.05) reversal of hypokalemia vs. 59% of patients on the placebo/hydrochlorothiazide regimen. The mean serum potassium concentration on 37.5 mg triamterene went from 3.2 ± 0.2 mEq/L at baseline to 3.7 ± 0.3 mEq/L at Week 4, a significantly greater (P<0.05) improvement than that achieved with placebo/hydrochlorothiazide (i.e., 3.2 ± 0.2 mEq/L at baseline and 3.5 ± 0.4 mEq/L at Week 4). Also, 51% of patients in the 37.5-mg triamterene group had an increase in serum potassium of ≥0.5 mEq/L at Week 4 vs. 33% in the placebo group. The 37.5-mg triamterene/25-mg hydrochlorothiazide regimen also maintained control of blood pressure; mean supine systolic blood pressure at Week 4 was 138 ± 21 mmHg while mean supine diastolic blood pressure was 87 ± 13 mmHg.
INDICATIONS AND USAGE
This fixed combination drug is not indicated for the initial therapy of edema or hypertension except in individuals in whom the development of hypokalemia cannot be risked.
DYAZIDE is indicated for the treatment of hypertension or edema in patients who develop hypokalemia on hydrochlorothiazide alone.
DYAZIDE is also indicated for those patients who require a thiazide diuretic and in whom the development of hypokalemia cannot be risked.
DYAZIDE may be used alone or as an adjunct to other antihypertensive drugs, such as beta-blockers. Since DYAZIDE may enhance the action of these agents, dosage adjustments may be necessary.
Usage in Pregnancy:
The routine use of diuretics in an otherwise healthy woman is inappropriate and exposes mother and fetus to unnecessary hazard. Diuretics do not prevent development of toxemia of pregnancy, and there is no satisfactory evidence that they are useful in the treatment of developed toxemia.
Edema during pregnancy may arise from pathological causes or from the physiologic and mechanical consequences of pregnancy. Diuretics are indicated in pregnancy when edema is due to pathologic causes, just as they are in the absence of pregnancy. Dependent edema in pregnancy resulting from restriction of venous return by the expanded uterus is properly treated through elevation of the lower extremities and use of support hose; use of diuretics to lower intravascular volume in this case is illogical and unnecessary. There is hypervolemia during normal pregnancy which is harmful to neither the fetus nor the mother (in the absence of cardiovascular disease), but which is associated with edema, including generalized edema in the majority of pregnant women. If this edema produces discomfort, increased recumbency will often provide relief. In rare instances this edema may cause extreme discomfort which is not relieved by rest. In these cases, a short course of diuretics may provide relief and may be appropriate.
CONTRAINDICATIONS
Antikaliuretic Therapy and Potassium Supplementation:
DYAZIDE should not be given to patients receiving other potassium-sparing agents such as spironolactone, amiloride, or other formulations containing triamterene. Concomitant potassium-containing salt substitutes should also not be used.
Potassium supplementation should not be used with DYAZIDE except in severe cases of hypokalemia. Such concomitant therapy can be associated with rapid increases in serum potassium levels. If potassium supplementation is used, careful monitoring of the serum potassium level is necessary.
Impaired Renal Function:
DYAZIDE is contraindicated in patients with anuria, acute and chronic renal insufficiency, or significant renal impairment.
WARNINGS
Hyperkalemia: Abnormal elevation of serum potassium levels (greater than or equal to 5.5 mEq/liter) can occur with all potassium-sparing diuretic combinations, including DYAZIDE. Hyperkalemia is more likely to occur in patients with renal impairment and diabetes (even without evidence of renal impairment) and in the elderly or severely ill. Since uncorrected hyperkalemia may be fatal, serum potassium levels must be monitored at frequent intervals especially in patients first receiving DYAZIDE, when dosages are changed, or with any illness that may influence renal function.
If hyperkalemia is suspected (warning signs include paresthesias, muscular weakness, fatigue, flaccid paralysis of the extremities, bradycardia, and shock), an electrocardiogram (ECG) should be obtained. However, it is important to monitor serum potassium levels because hyperkalemia may not be associated with ECG changes.
If hyperkalemia is present, DYAZIDE should be discontinued immediately and a thiazide alone should be substituted. If the serum potassium exceeds 6.5 mEq/L, more vigorous therapy is required. The clinical situation dictates the procedures to be employed. These include the intravenous administration of calcium chloride solution, sodium bicarbonate solution, and/or the oral or parenteral administration of glucose with a rapid-acting insulin preparation. Cationic exchange resins such as sodium polystyrene sulfonate may be orally or rectally administered. Persistent hyperkalemia may require dialysis.
The development of hyperkalemia associated with potassium-sparing diuretics is accentuated in the presence of renal impairment (see CONTRAINDICATIONS). Patients with mild renal functional impairment should not receive this drug without frequent and continuing monitoring of serum electrolytes. Cumulative drug effects may be observed in patients with impaired renal function. The renal clearances of hydrochlorothiazide and the pharmacologically active metabolite of triamterene, the sulfate ester of hydroxytriamterene, have been shown to be reduced and the plasma levels increased following administration of DYAZIDE to elderly patients and patients with impaired renal function.
Hyperkalemia has been reported in diabetic patients with the use of potassium-sparing agents even in the absence of apparent renal impairment. Accordingly, serum electrolytes must be frequently monitored if DYAZIDE is used in diabetic patients.
Metabolic or Respiratory Acidosis:
Potassium-sparing therapy should also be avoided in severely ill patients in whom respiratory or metabolic acidosis may occur. Acidosis may be associated with rapid elevations in serum potassium levels. If DYAZIDE is employed, frequent evaluations of acid/base balance and serum electrolytes are necessary.
Acute Myopia and Secondary Angle-Closure Glaucoma:
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
PRECAUTIONS
Diabetes:
Caution should be exercised when administering DYAZIDE to patients with diabetes, since thiazides may cause hyperglycemia, glycosuria, and alter insulin requirements in diabetes. Also, diabetes mellitus may become manifest during thiazide administration.
Impaired Hepatic Function:
Thiazides should be used with caution in patients with impaired hepatic function. They can precipitate hepatic coma in patients with severe liver disease. Potassium depletion induced by the thiazide may be important in this connection. Administer DYAZIDE cautiously and be alert for early signs of impending coma such as confusion, drowsiness, and tremor; if mental confusion increases, discontinue DYAZIDE for a few days. Attention must be given to other factors that may precipitate hepatic coma, such as blood in the gastrointestinal tract or pre-existing potassium depletion.
Hypokalemia:
Hypokalemia is uncommon with DYAZIDE; but, should it develop, corrective measures should be taken such as potassium supplementation or increased intake of potassium-rich foods. Institute such measures cautiously with frequent determinations of serum potassium levels, especially in patients receiving digitalis or with a history of cardiac arrhythmias. If serious hypokalemia (serum potassium less than 3.0 mEq/L) is demonstrated by repeat serum potassium determinations, DYAZIDE should be discontinued and potassium chloride supplementation initiated. Less serious hypokalemia should be evaluated with regard to other coexisting conditions and treated accordingly.
Electrolyte Imbalance:
Electrolyte imbalance, often encountered in conditions such as heart failure, renal disease, or cirrhosis of the liver, may also be aggravated by diuretics and should be considered during therapy with DYAZIDE when using high doses for prolonged periods or in patients on a salt-restricted diet. Serum determinations of electrolytes should be performed and are particularly important if the patient is vomiting excessively or receiving fluids parenterally. Possible fluid and electrolyte imbalance may be indicated by warning signs such as: dry mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pain or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal symptoms.
Hypochloremia:
Although any chloride deficit is generally mild and usually does not require specific treatment except under extraordinary circumstances (as in liver disease or renal disease), chloride replacement may be required in the treatment of metabolic alkalosis. Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is water restriction, rather than administration of salt, except in rare instances when the hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
Renal Stones:
Triamterene has been found in renal stones in association with the other usual calculus components. DYAZIDE should be used with caution in patients with a history of renal stones.
Laboratory Tests:
Serum Potassium:
The normal adult range of serum potassium is 3.5 to 5.0 mEq/L with 4.5 mEq often being used for a reference point. If hypokalemia should develop, corrective measures should be taken such as potassium supplementation or increased dietary intake of potassium-rich foods.
Institute such measures cautiously with frequent determinations of serum potassium levels. Potassium levels persistently above 6 mEq/L require careful observation and treatment. Serum potassium levels do not necessarily indicate true body potassium concentration. A rise in plasma pH may cause a decrease in plasma potassium concentration and an increase in the intracellular potassium concentration. Discontinue corrective measures for hypokalemia immediately if laboratory determinations reveal an abnormal elevation of serum potassium.
Discontinue DYAZIDE and substitute a thiazide diuretic alone until potassium levels return to normal.
Serum Creatinine and Blood Urea Nitrogen:
DYAZIDE may produce an elevated blood urea nitrogen (BUN) level, creatinine level, or both. This apparently is secondary to a reversible reduction of glomerular filtration rate or a depletion of intravascular fluid volume (prerenal azotemia) rather than renal toxicity; levels usually return to normal when DYAZIDE is discontinued. If azotemia increases, discontinue DYAZIDE. Periodic BUN or serum creatinine determinations should be made, especially in elderly patients and in patients with suspected or confirmed renal insufficiency.
Serum Protein-Bound Iodine:
Thiazide may decrease serum protein-bound iodine (PBI) levels without sign of thyroid disturbance.
Parathyroid Function:
Thiazides should be discontinued before carrying out tests for parathyroid function. Calcium excretion is decreased by thiazides. Pathologic changes in the parathyroid glands with hypercalcemia and hypophosphatemia have been observed in a few patients on prolonged thiazide therapy. The common complications of hyperparathyroidism such as bone resorption and peptic ulceration have not been seen.
Drug Interactions:
Angiotensin-Converting Enzyme Inhibitors:
Potassium-sparing agents should be used with caution in conjunction with angiotensin-converting enzyme (ACE) inhibitors due to an increased risk of hyperkalemia.
Oral Hypoglycemic Drugs:
Concurrent use with chlorpropamide may increase the risk of severe hyponatremia.
Nonsteroidal Anti-inflammatory Drugs:
A possible interaction resulting in acute renal failure has been reported in a few patients on DYAZIDE when treated with indomethacin, a nonsteroidal anti-inflammatory agent. Caution is advised in administering nonsteroidal anti-inflammatory agents with DYAZIDE.
Lithium:
Lithium generally should not be given with diuretics because they reduce its renal clearance and increase the risk of lithium toxicity. Read prescribing information for lithium preparations before use of such concomitant therapy with DYAZIDE.
Surgical Considerations:
Thiazides have been shown to decrease arterial responsiveness to norepinephrine (an effect attributed to loss of sodium). This diminution is not sufficient to preclude effectiveness of the pressor agent for therapeutic use. Thiazides have also been shown to increase the paralyzing effect of nondepolarizing muscle relaxants such as tubocurarine (an effect attributed to potassium loss); consequently, caution should be observed in patients undergoing surgery.
Other Considerations:
Concurrent use of hydrochlorothiazide with amphotericin B or corticosteroids or corticotropin (ACTH) may intensify electrolyte imbalance, particularly hypokalemia, although the presence of triamterene minimizes the hypokalemic effect.
Thiazides may add to or potentiate the action of other antihypertensive drugs. See INDICATIONS AND USAGE for concomitant use with other antihypertensive drugs.
The effect of oral anticoagulants may be decreased when used concurrently with hydrochlorothiazide; dosage adjustments may be necessary.
DYAZIDE may raise the level of blood uric acid; dosage adjustments of antigout medication may be necessary to control hyperuricemia and gout.
The following agents given together with triamterene may promote serum potassium accumulation and possibly result in hyperkalemia because of the potassium-sparing nature of triamterene, especially in patients with renal insufficiency: blood from blood bank (may contain potassium up to 30 mEq/L of plasma or up to 65 mEq/L of whole blood when stored for more than 10 days); low-salt milk (may contain potassium up to 60 mEq/L); potassium-containing medications (such as parenteral penicillin G potassium); salt substitutes (most contain substantial amounts of potassium).
Exchange resins, such as sodium polystyrene sulfonate, whether administered orally or rectally, reduce serum potassium levels by sodium replacement of the potassium; fluid retention may occur in some patients because of the increased sodium intake.
Chronic or overuse of laxatives may reduce serum potassium levels by promoting excessive potassium loss from the intestinal tract; laxatives may interfere with the potassium-retaining effects of triamterene.
The effectiveness of methenamine may be decreased when used concurrently with hydrochlorothiazide because of alkalinization of the urine.
Drug/Laboratory Test Interactions:
Triamterene and quinidine have similar fluorescence spectra; thus, DYAZIDE will interfere with the fluorescent measurement of quinidine.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Carcinogenesis:
Long-term studies have not been conducted with DYAZIDE (the triamterene/hydrochlorothiazide combination) or with triamterene alone.
Hydrochlorothiazide:
Two-year feeding studies in mice and rats, conducted under the auspices of the National Toxicology Program (NTP) treated mice and rats with doses of hydrochlorothiazide up to 600 and 100 mg/kg/day, respectively. On a body-weight basis, these doses are 600 times (in mice) and 100 times (in rats) the maximum recommended human dose (MRHD) for the hydrochlorothiazide component of DYAZIDE at 50 mg/day (or 1.0 mg/kg/day based on 50-kg individuals). On the basis of body surface area, these doses are 56 times (in mice) and 21 times (in rats) the MRHD. These studies uncovered no evidence of carcinogenic potential of hydrochlorothiazide in rats or female mice, but there was equivocal evidence of hepatocarcinogenicity in male mice.
Mutagenesis:
Studies of the mutagenic potential of DYAZIDE (the triamterene/hydrochlorothiazide combination) or of triamterene alone have not been performed.
Hydrochlorothiazide:
Hydrochlorothiazide was not genotoxic in in vitro assays using strains TA 98, TA 100, TA 1535, TA 1537, and TA 1538 of Salmonella typhimurium (the Ames test); in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations; or in in vivo assays using mouse germinal cell chromosomes, Chinese hamster bone marrow chromosomes, and the Drosophila sex-linked recessive lethal trait gene. Positive test results were obtained in the in vitro CHO Sister Chromatid Exchange (clastogenicity) test and in the mouse Lymphoma Cell (mutagenicity) assays, using concentrations of hydrochlorothiazide of 43 to 1,300 mcg/mL. Positive test results were also obtained in the Aspergillus nidulans nondisjunction assay, using an unspecified concentration of hydrochlorothiazide.
Impairment of Fertility:
Studies of the effects of DYAZIDE (the triamterene/hydrochlorothiazide combination) or of triamterene alone on animal reproductive function have not been conducted.
Hydrochlorothiazide:
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 and 4 mg/kg/day, respectively, prior to mating and throughout gestation. Corresponding multiples of the MRHD are 100 (mice) and 4 (rats) on the basis of body weight and 9.4 (mice) and 0.8 (rats) on the basis of body surface area.
Pregnancy:
Teratogenic Effects:
DYAZIDE:
Animal reproduction studies to determine the potential for fetal harm by DYAZIDE have not been conducted. However, a One Generation Study in the rat approximated composition of DYAZIDE by using a 1:1 ratio of triamterene to hydrochlorothiazide (30:30 mg/kg/day); there was no evidence of teratogenicity at those doses which were, on a body-weight basis, 15 and 30 times, respectively, the MRHD, and on the basis of body surface area, 3.1 and 6.2 times, respectively, the MRHD.
The safe use of DYAZIDE in pregnancy has not been established since there are no adequate and well-controlled studies with DYAZIDE in pregnant women. DYAZIDE should be used during pregnancy only if the potential benefit justifies the risk to the fetus.
Triamterene:
Reproduction studies have been performed in rats at doses as high as 20 times the MRHD on the basis of body weight and 6 times the human dose on the basis of body surface area without evidence of harm to the fetus due to triamterene.
Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Hydrochlorothiazide:
Hydrochlorothiazide was orally administered to pregnant mice and rats during respective periods of major organogenesis at doses up to 3,000 and 1,000 mg/kg/day, respectively. At these doses, which are multiples of the MRHD equal to 3,000 for mice and 1,000 for rats, based on body weight, and equal to 282 for mice and 206 for rats, based on body surface area, there was no evidence of harm to the fetus.
There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nonteratogenic Effects:
Thiazides and triamterene have been shown to cross the placental barrier and appear in cord blood. The use of thiazides and triamterene in pregnant women requires that the anticipated benefit be weighed against possible hazards to the fetus. These hazards include fetal or neonatal jaundice, pancreatitis, thrombocytopenia, and possible other adverse reactions which have occurred in the adult.
ADVERSE REACTIONS
Adverse effects are listed in decreasing order of severity.
Hypersensitivity:
Anaphylaxis, rash, urticaria, subacute cutaneous lupus erythematosus-like reactions, photosensitivity.
Metabolic:
Diabetes mellitus, hyperkalemia, hypokalemia, hyponatremia, acidosis, hypercalcemia, hyperglycemia, glycosuria, hyperuricemia, hypochloremia.
Gastrointestinal:
Jaundice and/or liver enzyme abnormalities, pancreatitis, nausea and vomiting, diarrhea, constipation, abdominal pain.
Renal:
Acute renal failure (one case of irreversible renal failure has been reported), interstitial nephritis, renal stones composed primarily of triamterene, elevated BUN and serum creatinine, abnormal urinary sediment.
Miscellaneous:
Impotence, sialadenitis.
Thiazides alone have been shown to cause the following additional adverse reactions:
Neonate and infancy:
Thrombocytopenia and pancreatitis−rarely, in newborns whose mothers have received thiazides during pregnancy.
Skin:
Erythema multiforme, including Stevens-Johnson syndrome; exfoliative dermatitis, including toxic epidermal necrolysis.
Postmarketing Experience:
Non-Melanoma Skin Cancer:
Hydrochlorothiazide is associated with an increased risk of non-melanoma skin cancer. In a study conducted in the Sentinel System, increased risk was predominantly for squamous cell carcinoma (SCC) and in white patients taking large cumulative doses. The increased risk for SCC in the overall population was approximately 1 additional case per 16,000 patients per year, and for white patients taking a cumulative dose of ≥50,000 mg the risk increase was approximately 1 additional SCC case for every 6,700 patients per year.
DOSAGE AND ADMINISTRATION
The usual dose of DYAZIDE is 1 or 2 capsules given once daily, with appropriate monitoring of serum potassium and of the clinical effect (see WARNINGS, Hyperkalemia).
OVERDOSAGE
Electrolyte imbalance is the major concern (see WARNINGS). Symptoms reported include: polyuria, nausea, vomiting, weakness, lassitude, fever, flushed face, and hyperactive deep tendon reflexes. If hypotension occurs, it may be treated with pressor agents such as levarterenol to maintain blood pressure. Carefully evaluate the electrolyte pattern and fluid balance. Induce immediate evacuation of the stomach through emesis or gastric lavage. There is no specific antidote.
Reversible acute renal failure following ingestion of 50 tablets of a product containing a combination of 50 mg triamterene and 25 mg hydrochlorothiazide has been reported.
Although triamterene is largely protein-bound (approximately 67%), there may be some benefit to dialysis in cases of overdosage.
HOW SUPPLIED
Capsules containing 25 mg hydrochlorothiazide and 37.5 mg triamterene supplied as follows:
Patient-Pak unit-of-use bottles of 100 NDC 0007-3650-22.
Store at controlled room temperature 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F). Protect from light. Dispense in a tight, light-resistant container.
Distributed by:
GlaxoSmithKline
Research Triangle Park, NC 27709
Trademarks are owned by or licensed to the GSK group of companies.
©2020 GSK group of companies or its licensor.
August 2020
DYZ:76PI
Principal Display Panel
NDC 0007-3650-22
DYAZIDE
25 MG HYDROCHLOROTHIAZIDE AND 37.5 MG TRIAMTERENE CAPSULES
100 Capsules
Rx only
Store at controlled room temperature 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F).
Do not accept if membrane seal under cap is missing or broken.
Protect from light. Dispense in a tight, light-resistant container.
Each capsule contains 25 mg hydrochlorothiazide and 37.5 mg triamterene.
DYAZIDE capsules meet Drug Release Test 3 as published in the current USP monograph for Triamterene and Hydrochlorothiazide Capsules.
Usual Dosage: 1 or 2 capsules once daily. See accompanying prescribing information.
Important: Use safety closures when dispensing this product unless otherwise directed by physician or requested by purchaser.
Trademarks are owned by or licensed to the GSK group of companies.
GlaxoSmithKline
RTP, NC 27709
Made in Canada
2018 GSK group of companies or its licensor.
- 62000000033258 Rev. 12/18
| DYAZIDE
hydrochlorothiazide and triamterene capsule |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - GlaxoSmithKline LLC (167380711) |