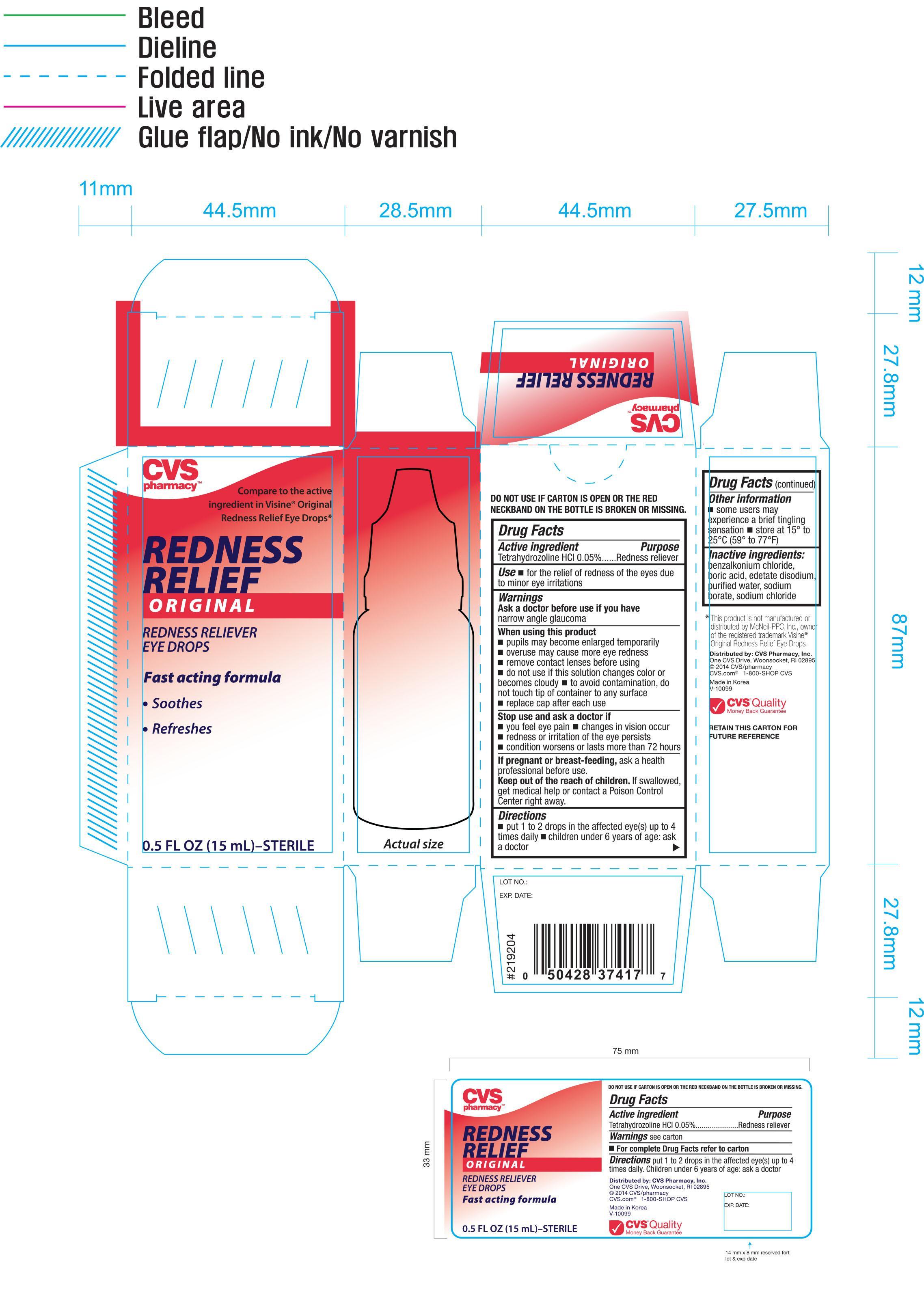
CVS REDNESS RELIEF ORIGINAL- tetrahydrozoline hydrochloride solution/ drops
CVS Pharmacy, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CVS Redness Relief Original Eye Drops 0.5 oz (37417)
When using this product
- pupils may become enlarged temporarily
- overuse may cause more eye redness
- remove contact lenses before using
- do not use if this solution changes color or becomes cloudy
- to avoid contamination, do not touch tip of container to any surface
- replace cap after each use
Stop use and ask a doctor if
- you feel eye pain
- changes in vision occur
- redness or irritation of the eye persists
- condtion worsens or lasts more than 72 hours
Keep out of the reach of children. If swallowed, get medical help or contact a Poision Control Center right away
Directions
- put 1 to 2 drops in the affected eye(s) up to 4 times daily
- children under 6 years of age: ask a doctor
Other information
- some users may experience a brief tingling sensation
- store at 15° to 25°C (59° to 77°F)
| CVS REDNESS RELIEF ORIGINAL
tetrahydrozoline hydrochloride solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CVS Pharmacy, Inc. (062312574) |
Revised: 8/2023
Document Id: 02ad267f-ab91-792c-e063-6394a90a5038
Set id: f68f7e67-5d47-4d8b-8620-bb1e2631740e
Version: 6
Effective Time: 20230811
CVS Pharmacy, Inc.